Abstract
Background
Although triglyceride-glucose (TyG) index is a reliable indicator of insulin resistance and cardiometabolic disease, its effectiveness in predicting mortality risk has not been adequately validated. We aimed to investigate the association between the TyG-related indices and all-cause and cause-specific mortality in the general population.
Methods
A total of 27,642 individuals were included from the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2018. Three indicators were constructed, including the TyG index, TyG combined with waist-to-height ratio (TyG-WHtR), and TyG combined with waist circumference (TyG-WC). Mortality data was acquired through the linkage of NHANES data with National Death Index records. Weighted Cox proportional hazards models were used to estimate the independent association between the TyG-related indices and mortality. Nonlinear associations were explored using restricted cubic splines.
Results
Multivariable adjusted models showed a progressive increase in all-cause and cause-specific mortality across quartiles of the TyG-related indices. Compared with the lowest quartile of the TyG index, the highest quartile had adjusted hazard ratios of 1.26 (95% CI 1.04–1.52) for all-cause mortality, 1.38 (1.04–1.74) for cardiovascular mortality, and 1.23 (1.01–1.50) for non-cardiovascular mortality, respectively. For the TyG-WHtR index, the corresponding hazard ratios were 1.60 (1.25–2.05), 1.86 (1.26–2.50), and 1.48 (1.10–1.99), respectively. For the TyG-WC index, the corresponding hazard ratios were 1.42 (1.11–1.75), 1.48 (1.04–1.96), and 1.38 (1.05–1.72), respectively. The associations between the three TyG-related indices and all-cause, cardiovascular and non-cardiovascular mortality were J-shaped. Interaction tests revealed significant effect modification by age, low-density lipoprotein cholesterol (LDL-C) level, and statin use (all P values < 0.05).
Conclusions
The TyG-related indices were independent predictors of all-cause and cause-specific mortality in the general population. Young individuals should be particularly vigilant, whereas low LDL-C levels and statin use are potentially protective.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02390-0.
Keywords: Triglyceride-glucose index, Waist-to-height ratio, Waist circumference, Anthropometric measure, All-cause mortality, Cause-specific mortality
Introduction
Reducing overall mortality and improving life expectancy are the overarching goals of the global public health sustainable development framework [1]. In recent decades, there has been steady progress in global population health and life expectancy. However, the outbreak of the COVID-19 pandemic reversed this trend, wiping out the advancements within just 2 years [1]. The development of effective, cost-efficient, and easily accessible prognostic biomarkers for the early identification of individuals at high risk of mortality and the design of intensive surveillance and tailored interventions to counteract the escalation in global mortality remains a challenging endeavor.
Insulin resistance (IR) is characterized by impaired insulin sensitivity and reduced glucose disposal capacity of insulin-targeted tissues, as evidenced by a shift of the insulin dose-response curve toward higher insulin concentrations [2]. Insulin resistance not only manifests as metabolic abnormalities, but also bears a high risk of multiple organ dysfunction and premature death [3, 4]. Several methods for quantifying insulin sensitivity in vivo, such as hyperinsulinaemic-euglycaemic clamp (HIEC), homeostasis model assessment for IR (HOMA-IR) and the quantitative insulin sensitivity checking index (QUICKI), are challenging in clinical application due to time-consuming, expensive, and procedurally cumbersome [5–7]. The TyG index is gaining popularity as a simple, convenient and cost-effective surrogate for the assessment of IR. Accumulating evidence supports a strong association between elevated TyG index and the incidence of new-onset diabetes, atherosclerotic cardiovascular disease (ASCVD), heart failure, and metabolic syndrome, as well as subsequent adverse clinical outcomes [8–12]. Epidemiological and genetic studies have shown that anthropometric measures reflecting central obesity, such as waist-to-height ratio (WHtR) and waist circumference (WC), are more strongly associated with significant cardiometabolic risk than traditional measures reflecting overall obesity with body mass index (BMI) [13, 14]. Therefore, combining the TyG index with these metrics (WHtR and WC) may provide additional predictive performance. Previous studies have shown that the TyG-WHtR and TyG-WC indices are superior to the TyG index alone in identifying the risk of cardiovascular disease risk and diabetes mellitus [15, 16]. However, previous studies have mainly focused on the impact of these indices on disease incidence and composite adverse endpoints, with few comprehensive data available on the relationship between the TyG, TyG-WHtR, and TyG-WC indicator and all-cause and detailed cause-specific mortality in the general population. In addition, other TyG-related indices, which derived from TyG index combined with other anthropometric measures reflecting central adiposity, including body roundness index (BRI), body shape index (BSI), weight-adjusted-waist index (WWI), relative fat mass (RFM), and conicity index (CI), have not been studied.
Therefore, we conducted a study utilizing NHANES data to examine the association of a series of TyG-related indices with all-cause and cause-specific mortality in the general population to address the current knowledge gap. This issue has important implications for the search for intervenable biomarkers for stratified prevention and targeted treatment to reduce overall mortality.
Methods
Study population
The datasets are derived from ten cycles of the NHANES database from 1999 to 2018. NHANES utilizes a stratified, multistage probability sampling design to systematically collect nutrition and health data from a nationally representative, non-institutionalized US civilian population [17]. The study was conducted in accordance with the principles of the Declaration of Helsinki. The Ethical Review Board of the National Center for Health Statistics granted ethics approval for each NHANES cycle. All individuals signed written informed consent. Of the 101,316 participants, 73,674 were excluded because they were younger than 18 years (n = 42,112), pregnant (n = 1596), lost to follow-up (n = 2874), had missing triglyceride or glucose measurements (n = 3608), or had fasting times of less than 8 h (n = 23,484). Finally, 27,642 individuals were included in the analysis of TyG index. In addition, due to missing data on waist circumference (WC) (n = 971) and height (n = 367), TyG-WHtR and TyG-WC analyses were available for 26,601 and 26,673 individuals, respectively (Additional file 1: Fig. S1).
Exposure
Fasting triglycerides (TG) and fasting blood glucose (FBG) were measured by enzymatic colorimetric assays using blood samples collected after at least 8 h of fasting. TG concentrations were measured using the Roche Modular P and Roche Cobas 6000 chemistry analyzers. FBG concentrations were measured using the Roche/Hitachi Cobas C 501 system. TyG-related indices were calculated as follows: TyG index = ln [TG(mg/dL)×FBG(mg/dL)/2], TyG-WHtR index = ln [TG(mg/dL)×FBG(mg/dL)/2]×WC/height, and TyG-WC index = ln [TG(mg/dL)×FBG(mg/dL)/2]×WC. Participants were divided into four groups according to the quartiles of the TyG-related indices, with the group in the first quartile serving as the reference.
Besides WhtR and WC, the TyG index was also analyzed in combination with other anthropometric measures reflecting central adiposity, including BRI, BSI, WWI, RFM, and CI. The calculation formulas are shown as: TyG-BRI index = TyG index×[364.2–365.5×[1−-(WC/2π)2] / (0.5×height2)1/2], TyG-BSI index = TyG index×[WC/(BMI2/3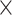 height1/2)], TyG-WWI index = TyG index×[WC×100/weight1/2], TyG-RFM index = TyG index×[64−(20×height/WC) + (12×sex), sex = 0 for males and 1 for females], and TyG-CI index = TyG index×[WC
height1/2)], TyG-WWI index = TyG index×[WC×100/weight1/2], TyG-RFM index = TyG index×[64−(20×height/WC) + (12×sex), sex = 0 for males and 1 for females], and TyG-CI index = TyG index×[WC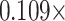 (
(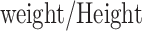 )1/2]. As a sensitivity analysis, the TyG index was examined in combination with anthropometric measures reflecting overall adiposity, including body mass index (BMI) and body surface area (BSA).
)1/2]. As a sensitivity analysis, the TyG index was examined in combination with anthropometric measures reflecting overall adiposity, including body mass index (BMI) and body surface area (BSA).
Clinical endpoints
Mortality data were obtained from the NHANES public-use linked mortality file as of December 31, 2019. This file was linked to the National Death Index (NDI) from the National Center for Health Statistics (NCHS) using a probability matching algorithm. Causes of death were examined according to ICD-10 (International Classification of Diseases, Tenth Revision) and the underlying classification of death was recoded. Cardiovascular mortality refers to deaths from heart diseases (054-068) and cerebrovascular diseases (070). Non-cardiovascular mortality refers to deaths from diseases other than cardiovascular diseases, including malignant neoplasms (019-043), diabetes mellitus (046), Alzheimer’s disease (052), respiratory diseases (076-078 and 082-086), renal diseases (097-101) and all residual causes.
Covariates
Demographic information was collected through a computer-assisted interview system, including age, sex, ethnicity, education level, marital status, and poverty income ratio (PIR). Lifestyle information and history of comorbidities were also collected, including smoking status, alcohol consumption, history of diabetes mellitus, ASCVD, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), cancer, and renal disease. Diabetes mellitus was defined as hemoglobin A1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL, or 2-h postprandial glucose ≥ 200 mg/dL, or use of insulin or oral hypoglycemic agents. ASCVD included coronary artery disease and stroke. Renal disease was determined by an estimated glomerular filtration rate (eGFR) < 60 mL/min. CHF and COPD were determined by physician diagnosis and prescription medication. Physical examinations, including blood pressure, waist circumference, body weight and height, were measured using standardized methods at the mobile examination center. Use of insulin or antihyperglycemic agents and statins was obtained from the Prescription Drug Questionnaire. In addition, serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), albumin, and eGFR were also selected as potential confounders.
Statistical analyses
All analyses were performed using R statistical software (R project, version 4.3.2) and EmpowerStats (X&Y Solutions, Inc., Boston, MA). Strata, primary sampling units, and probability weights were applied to account for the complex design of clustering, stratification, nonresponse probability, and population oversampling in NHANES. Continuous variables were reported as mean ± standard deviation (SD) or median (interquartile range). Categorical variables were reported as percentages. Differences in baseline characteristics were compared by weighted one-way analysis of variance for continuous variables and weighted chi-squared test for categorical variables. Correlations between different TyG-related indices were tested by linear regression fitting and Pearson correlation.
Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) between categorical TyG, TyG-WHtR, and TyG-WC and all-cause and cause-specific mortality. P-values for trends were tested by treating the quartile level as an ordinal variable. The TyG-related indices were also examined as continuous variables to calculate the effect size of per unit increase. In addition, to limit the influence of extreme values, exposure variables were further standardized to z-scores, indicating the effect size per SD increase. The proportional hazards assumption was assessed using the Schoenfeld residuals test, and no violations were found. Models were adjusted for age, sex, ethnicity, systolic blood pressure, body mass index (BMI), smoking status, alcohol consumption, diabetes mellitus, ASCVD, COPD, CHF, renal disease, cancer, TC, HDL-C, LDL-C, albumin, eGFR, statin use, and insulin or antihyperglycemic agents. To address the issue of collinearity, the variance inflation factors (VIF) of the covariates were examined and only those with VIF < 10 were included in the model. Missing data for covariates including systolic blood pressure (n = 1255, 4.5%), BMI (n = 453, 1.6%), and LDL-C (n = 581, 2.1%) were imputed using chained equation multiple imputation method. Stratified analyses were performed, and interactions were examined using the Wald tests to account for potential effect modification. HRs and 95% CIs between continuous TyG, TyG-WHtR, and TyG-WC and subcategories of cause-specific mortality were estimated, while ensuring similar levels of adjustment.
Restricted cubic splines were used to visualize the dose-response relationship between TyG, TyG-WHtR, and TyG-WC and mortality. The number of knots was selected according to the minimization of Akaike’s information criterion to balance optimal fit and overfitting. If the association was nonlinear, possible threshold inflection points were estimated and a two-piecewise Cox proportional hazards model for both sides of the inflection point was constructed.
Several sensitivity analyses were performed to strengthen our findings. We excluded individuals with less than 2 years of follow-up to account for potential reverse causality. We examined the association between TyG-related indices and mortality in individuals with and without diabetes, in those < 65 years and ≥ 65 years, in those with different LDL-C levels (< 2.6 and ≥ 2.6 mmol/L), and in those taking or not taking statins. We analyzed men and women separately to determine whether there was a sex difference. In addition, we made additional adjustments for socioeconomic factors to demonstrate the robustness of the model. The cumulative incidence of all-cause mortality across quartiles of TyG-related indices was estimated using the Kaplan-Meier method and compared using the log-rank test. Cumulative incidence of cause-specific mortality was estimated using the Fine and Gray competing risks model (which treats death from another cause as a competing risk) and compared by Gray’s test. We also assessed the predictive power of TyG, TyG-WHtR, and TyG-WC indices for all-cause and cause-specific mortality using receiver operating characteristic curves (ROCs). Finally, we used linear regression fitting and Pearson’s correlation to test the correlation between TyG-related indices and the homeostasis model assessment of insulin resistance index (HOMA-IR).
Results
Of the 27,642 individuals included in the analysis, 13,870 (50.2%) were male, the mean age was 47.4 years (SD 19.2), and 11,893 (43.0%) were non-Hispanic white. During a median follow-up of 10.2 years, 4074 (14.7%) all-cause deaths were recorded, of which 1296 (4.7%) were cardiovascular deaths and 2778 (10.0%) were non-cardiovascular deaths. Median TG and FBG were 1.17 mmol/L (IQR 0.81–1.73) and 5.50 mmol/L (IQR 5.51–6.05), respectively. The TyG index ranged from 5.75 to 13.25, with a mean value of 8.55 (SD 0.69). From quartile 1 to quartile 4, participants’ age, male proportion, smoker proportion, prevalence of diabetes, ASCVD, cancer, COPD, CHF and renal disease, and levels of TC and LDL-C gradually increased, while BMI, HDL-C level and eGFR gradually decreased (Table 1). The mean values of TyG-WHtR and TyG-WC were 5.01 (SD 1.06) and 838.9 (SD 177.5), respectively. Similar trends were observed for the quartiles of TyG-WHtR and TyG-WC (Additional file 1: Tables S1 and S2). There are significant positive correlations between the three TyG-related indices, with correlation coefficients ranging from 0.70 to 0.95 (Additional file 1: Fig. S2).
Table 1.
Baseline characteristics of individuals by quartiles of TyG index
| Quartile 1 (5.75–8.06) |
Quartile 2 (8.07–8.49) |
Quartile 3 (8.50–8.94) |
Quartile 4 (8.95–13.25) |
P value | |
|---|---|---|---|---|---|
| N | 6797 | 7012 | 6879 | 6954 | |
| Age, years | 38.0 ± 17.7 | 46.9 ± 19.6 | 50.8 ± 18.6 | 53.7 ± 16.8 | < 0.0001 |
| Male, n (%) | 2887 (39.8) | 3435 (48.1) | 3592 (52.2) | 3956 (58.1) | < 0.0001 |
| Ethnicity, n (%) | < 0.0001 | ||||
| Non-Hispanic White | 2492 (63.1) | 2987 (67.7) | 3170 (70.1) | 3244 (71.0) | |
| Non-Hispanic Black | 2248 (18.0) | 1651 (12.3) | 1071 (7.7) | 820 (6.0) | |
| Hispanic | 1462 (12.3) | 1774 (13.4) | 2014 (15.3) | 2282 (16.1) | |
| Other | 595 (6.6) | 600 (6.7) | 624 (6.9) | 608 (6.9) | |
| Systolic blood pressure, mmHg | 118 ± 18 | 124 ± 20 | 127 ± 20 | 131 ± 20 | < 0.0001 |
| Body mass index, kg/m2 | 31.3 ± 6.7 | 29.9 ± 6.8 | 28.2 ± 6.5 | 26.4 ± 6.0 | < 0.0001 |
| Smoking status, n (%) | < 0.0001 | ||||
| Never smoker | 4636 (64.1) | 4134 (56.4) | 3706 (51.6) | 3373 (47.3) | |
| Former smoker | 1026 (17.5) | 1477 (22.3) | 1765 (26.8) | 2042 (29.5) | |
| Current smoker | 1135 (18.5) | 1401 (21.3) | 1408 (21.6) | 1539 (23.3) | |
| Alcohol consumption, n (%) | 0.002 | ||||
| Never | 3008 (35.8) | 2907 (35.7) | 2823 (35.6) | 2872 (37.3) | |
| Less than once a week | 3646 (61.4) | 3888 (59.6) | 3830 (60.1) | 3878 (58.7) | |
| More than once a week | 143 (2.9) | 217 (4.7) | 226 (4.3) | 204 (3.9) | |
| Total cholesterol, mmol/L | 4.49 ± 0.90 | 4.89 ± 0.98 | 5.14 ± 1.02 | 5.45 ± 1.23 | < 0.0001 |
| HDL cholesterol, mmol/L | 1.59 ± 0.42 | 1.45 ± 0.40 | 1.32 ± 0.35 | 1.13 ± 0.30 | < 0.0001 |
| LDL cholesterol, mmol/L | 2.60 ± 0.77 | 2.99 ± 0.87 | 3.18 ± 0.93 | 3.14 ± 1.02 | < 0.0001 |
| Triglycerides, mmol/L | 0.62 (0.52–0.72) | 0.97 (0.87–1.09) | 1.42 (1.26–1.60) | 2.30 (1.92–2.99) | < 0.0001 |
| Glucose, mmol/L | 4.83 ± 0.51 | 5.14 ± 0.68 | 5.44 ± 0.96 | 6.86 ± 3.05 | |
| Albumin, g/L | 42.9 ± 3.5 | 42.4 ± 3.4 | 42.5 ± 3.4 | 42.8 ± 3.4 | < 0.0001 |
| eGFR, mL/min | 106.4 ± 24.1 | 97.9 ± 25.7 | 94.7 ± 25.5 | 92.3 ± 25.5 | < 0.0001 |
| Diabetes mellitus, n(%) | 608 (7.6) | 855 (9.9) | 1330 (15.3) | 2765 (33.6) | < 0.0001 |
| ASCVD, n(%) | 260 (3.2) | 495 (5.4) | 669 (7.7) | 899 (11.3) | < 0.0001 |
| COPD, n (%) | 244 (4.0) | 386 (5.9) | 430 (6.3) | 559 (8.5) | < 0.0001 |
| Cancer, n (%) | 344 (5.8) | 550 (8.0) | 649 (9.4) | 731 (10.9) | < 0.0001 |
| Chronic heart failure, n (%) | 84 (1.0) | 185 (1.8) | 223 (2.3) | 337 (4.1) | < 0.0001 |
| Chronic renal disease (eGFR < 60 mL/min), n (%) | 246 (2.4) | 508 (4.9) | 646 (6.6) | 762 (8.4) | < 0.0001 |
| Statin use, n (%) | 428 (5.7) | 900 (11.9) | 1184 (16.1) | 1671 (23.2) | < 0.0001 |
| Insulin or antihyperglycemic agents | 151 (1.5) | 315 (2.8) | 578 (5.7) | 1568 (18.5) | < 0.0001 |
| Education level, n (%) | < 0.0001 | ||||
| Under high school | 1178 (12.8) | 1695 (15.8) | 1902 (18.9) | 2262 (21.2) | |
| High school graduate | 1230 (19.7) | 1512 (23.5) | 1566 (24.5) | 1623 (26.5) | |
| Above high school/unknown | 4389 (67.5) | 3805 (60.8) | 3411 (56.5) | 3069 (52.3) | |
| Marital status, n (%) | < 0.0001 | ||||
| Married/cohabiting | 3153 (55.1) | 3826 (60.7) | 4092 (62.7) | 4271 (64.8) | |
| Separated/divorced/widowed | 975 (13.3) | 1392 (16.4) | 1485 (18.6) | 1724 (21.3) | |
| Never married/unknown | 2669 (31.6) | 1794 (23.0) | 1302 (18.7) | 959 (13.9) | |
| Poverty income ratio (PIR) | 2.1 (1.1−4.0) | 2.1 (1.1–4.1) | 2.1 (1.1−4.0) | 1.9 (1.1–3.6) | 0.002 |
| All-cause mortality | 524 (5.5) | 1005 (10.0) | 1158 (12.1) | 1387 (15.9) | < 0.0001 |
| Cardiovascular mortality | 144 (1.3) | 318 (3.0) | 367 (3.6) | 467 (5.1) | < 0.0001 |
| Non-cardiovascular mortality | 380 (4.2) | 687 (7.0) | 791 (8.5) | 920 (10.8) | < 0.0001 |
Continuous variables are presented as means (standard deviations) or medians (interquartile ranges), and categorical variables are presented as n (%). N, number of subjects; %, weighted percentage. PIR is calculated by dividing family income by family size, year, and geographic location, as measured by the Department of Health and Human Services. HDL high-density lipoprotein, LDL low-density lipoprotein, ASCVD atherosclerotic cardiovascular disease, COPD chronic obstructive pulmonary disease
Association between TyG-related indices and all-cause and cause-specific mortality
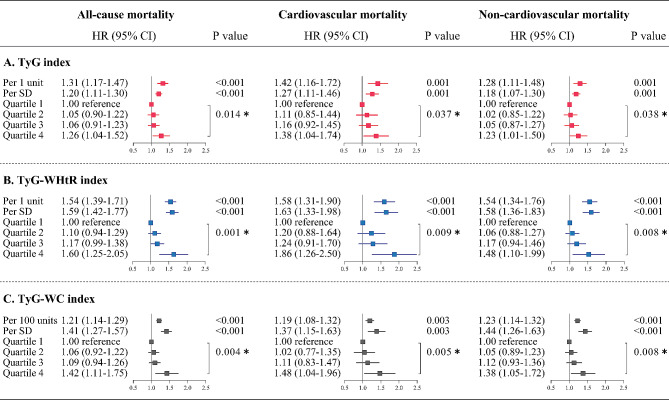
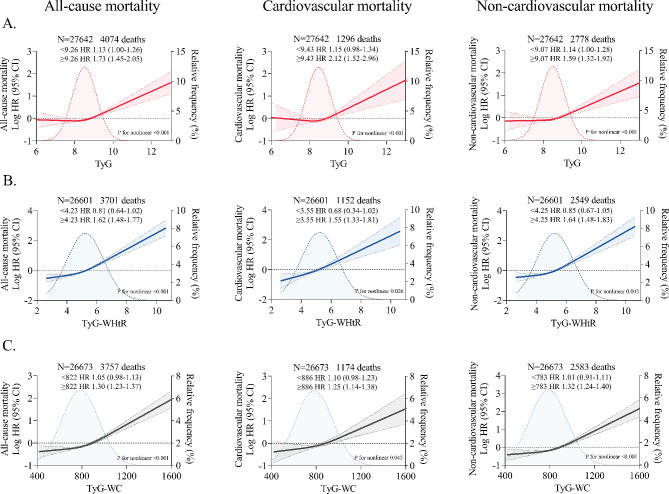
After adjustment for potential confounders, a stepwise incremental association was observed between increasing TyG-related indices and risk of death (P-value for trend < 0.05). Specifically, the adjusted hazard ratios for the TyG index quartile 4 vs. quartile 1 were 1.26 (95% CI 1.04–1.52) for all-cause mortality, 1.38 (1.04–1.74) for cardiovascular mortality, and 1.23 (1.01–1.50) for non-cardiovascular mortality. For the TyG-WHtR index, the corresponding hazard ratios were 1.60 (1.25–2.05), 1.86 (1.26–2.50), and 1.48 (1.10–1.99), respectively. For the TyG-WC index, the corresponding hazard ratios were 1.42 (1.11–1.75), 1.48 (1.04–1.96), and 1.38 (1.05–1.72), respectively. Results were consistent when TyG, TyG-WHtR, and TyG-WC indices were analyzed as continuous functions (each unit and each SD) (Fig. 1). On continuous scales, the TyG index was associated with the risk of death in a J-shaped manner, with inflection points for all-cause, cardiovascular, and non-cardiovascular deaths at TyG index of 9.26, 9.43, and 9.07, respectively. The risk of death remained almost constant until the inflection point, after which it increased sharply. Similar nonlinear trends persisted in the visualized relationships between TyG-WHtR and TyG-WC indices and mortality, with the corresponding inflection points occurring at TyG-WHtR index of 4.23, 3.55, and 4.25, and TyG-WC index of 822, 886, and 783, respectively (Fig. 2).
Fig. 1.
Cox proportional hazards regression analyses for the association of TyG-related indices with all-cause and cause-specific mortality. All-cause and cause-specific mortality for quartiles of A TyG index, B TyG-WHtR index, and C TyG-WC index. Adjusted for age, sex, ethnicity, BMI, systolic blood pressure, smoking status, alcohol consumption, diabetes mellitus, ASCVD, COPD, chronic heart failure, chronic renal disease, cancer, total cholesterol, HDL-C, LDL-C, albumin, eGFR, statin use, and insulin or antihyperglycemic drugs. ∗P value for trend. Effect sizes for per SD and per 1 unit or per 100 units increase in TyG-related indices were also shown separately. BMI body mass index, ASCVD atherosclerotic cardiovascular disease, COPD chronic obstructive pulmonary disease, HDL high-density lipoprotein, LDL low-density lipoprotein
Fig. 2.
Restricted cubic spline curve for the association of TyG-related indices with all-cause and cause-specific mortality. All-cause and cause-specific mortality for A TyG index, B TyG-WHtR index, and C TyG-WC index. Solid lines represent hazard ratios, and dashed lines represent 95% confidence intervals. Adjusted for age, sex, ethnicity, BMI, systolic blood pressure, smoking status, alcohol consumption, diabetes mellitus, ASCVD, COPD, chronic heart failure, chronic renal disease, cancer, total cholesterol, HDL-C, LDL-C, albumin, eGFR, statin use, and insulin or antihyperglycemic drugs. The shaded areas in the background show the distribution of TyG-related indices in the population. Two-piece Cox proportional hazards models were used to estimate the risk inflection point, and effect sizes for per 1 unit (TyG index and TyG-WHtR index) or per 100 units (TyG-WC index) increase in TyG-related indices before and after the inflection point were shown separately
Subgroup analysis of association between TyG-related indices and mortality
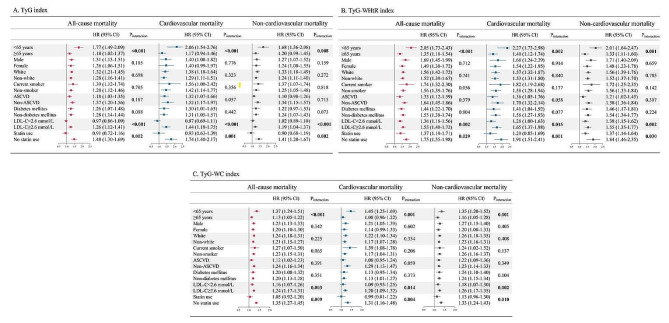
In subgroup analyses of the TyG index, significant interactions were observed between the TyG index and age, LDL-C level, and statin use on all-cause and cause-specific mortality, with the association being stronger in younger individuals, those with LDL-C ≥ 2.6 mmol/L, and those who were not taking statins. Subgroup analyses of the TyG-WHtR and TyG-WC indices also showed similar effect modification, with a higher risk of mortality in young individuals, those with high LDL-C levels, and those in statin-naive individuals (all p values for interactions < 0.05). No convincing evidence of an interaction was found in the groups stratified by sex, ethnicity, smoking status, ASCVD and diabetes mellitus (Fig. 3).
Fig. 3.
Stratified analyses of the associations between TyG-related indices and mortality. All-cause and cause-specific mortality in different strata as functions of 1-unit increase in A TyG index and B TyG-WHtR index or a 100-unit increase in C TyG-WC index. Adjusted for age, sex, ethnicity, BMI, systolic blood pressure, smoking status, alcohol consumption, diabetes mellitus, ASCVD, COPD, chronic heart failure, chronic renal disease, cancer, total cholesterol, HDL-C, LDL-C, albumin, eGFR, statin use, and insulin or antihyperglycemic drugs
Association between TyG-related indices and cause-specific mortality subcategories
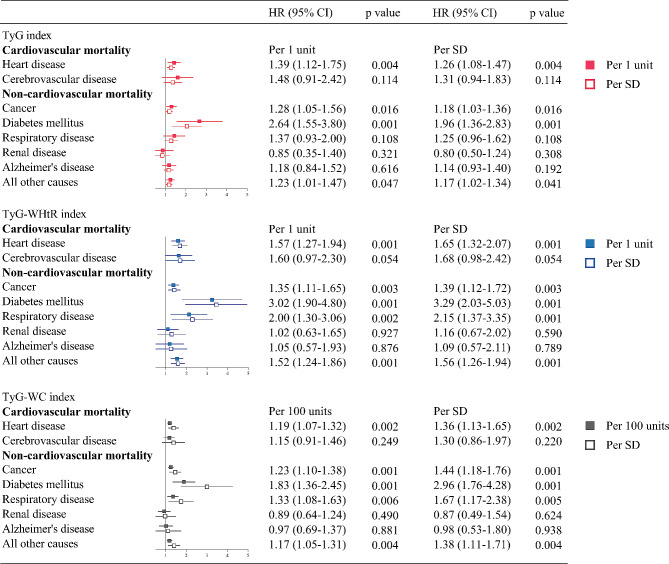
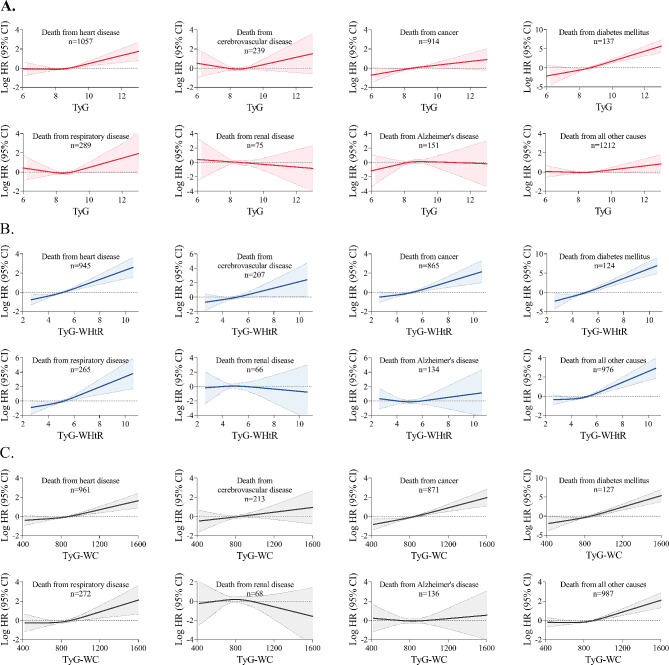
Multivariable adjusted Cox proportional hazards models showed that elevated TyG, TyG-WHtR, and TyG-WC indices were associated with increased hazard ratios for death from diabetes mellitus, heart disease, cancer, and all other residual causes. In addition, elevated TyG-WHtR, and TyG-WC indices were also associated with increased hazard ratios for death from respiratory disease. There was no compelling evidence that elevated TyG, TyG-WHtR, and TyG-WC indices were associated with increased hazard ratios for death from cerebrovascular disease, chronic renal disease, and Alzheimer’s disease (Fig. 4). Restricted cubic spline curves showed consistent results (Fig. 5).
Fig. 4.
Cox proportional hazards regression analyses for the association of TyG-related indices with cause-specific mortality subcategories. Adjusted for age, sex, ethnicity, BMI, systolic blood pressure, smoking status, alcohol consumption, diabetes mellitus, ASCVD, COPD, chronic heart failure, chronic renal disease, cancer, total cholesterol, HDL-C, LDL-C, albumin, eGFR, statin use, and insulin or antihyperglycemic drugs
Fig. 5.
Restricted cubic spline curve for the association of TyG-related indices with cause-specific mortality subcategories. A TyG index, B TyG-WHtR index, and C TyG-WC index. Solid lines represent hazard ratios, and dashed lines represent 95% confidence intervals. Adjusted for age, sex, ethnicity, BMI, systolic blood pressure, smoking status, alcohol consumption, diabetes mellitus, ASCVD, COPD, chronic heart failure, chronic renal disease, cancer, total cholesterol, HDL-C, LDL-C, albumin, eGFR, statin use, and insulin or antihyperglycemic drugs
Association between other TyG-derived indices and all-cause and cause-specific mortality
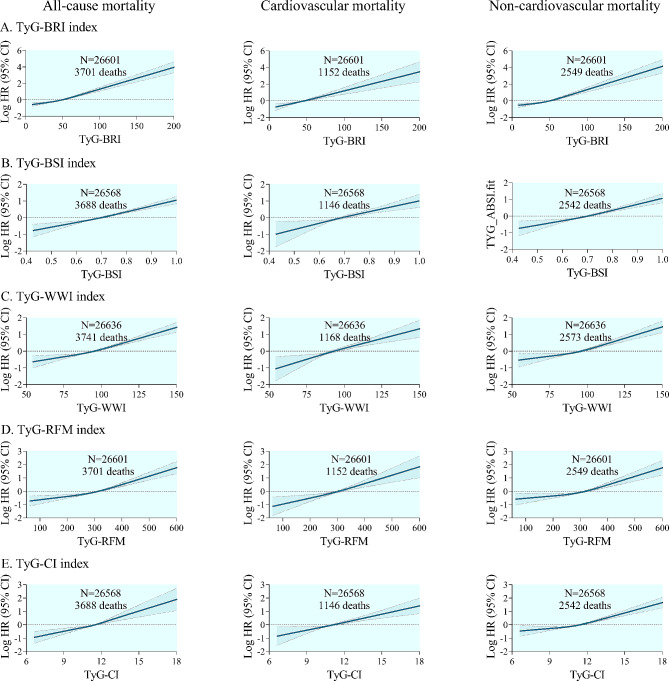
Restricted cubic spline curves showed significant positive dose-response relationships between other TyG-derived indices (including TyG-BRI, TyG-BSI, TyG-WWI, TyG-RFM, and TyG-CI) and all-cause and cause-specific mortality (Fig. 6). Notably, the anthropometric measures in all of these derived indices reflect central obesity, and their sources of calculation include a key component, waist circumference. However, when anthropometric indicators reflecting overall obesity are examined, such as TyG-BMI and TyG-BSA, these monotonic positive associations appear to be absent (Additional file 1: Fig. S3).
Fig. 6.
Restricted cubic spline curve for the association of other TyG-derived indices with all-cause and cause-specific mortality. All-cause and cause-specific mortality for A TyG-BRI index, B TyG-BSI index, C TyG-WWI index, D TyG-RFM index, and E TyG-CI index. Solid lines represent hazard ratios, and dashed lines represent 95% confidence intervals. Adjusted for age, sex, ethnicity, BMI, systolic blood pressure, smoking status, alcohol consumption, diabetes mellitus, ASCVD, COPD, chronic heart failure, chronic renal disease, cancer, total cholesterol, HDL-C, LDL-C, albumin, eGFR, statin use, and insulin or antihyperglycemic drugs. BRI body roundness index, BSI body shape index, WWI weight-adjusted-waist index, RFM relative fat mass, CI conicity index
Sensitivity analyses
Results remained robust when individuals with less than 2 years of follow-up were excluded (Additional file 1: Fig. S4). Results were consistent regardless of the presence or absence of a diagnosis of diabetes mellitus at baseline (Additional file 1: Figs. S5 and S6). A trend toward stronger associations between TyG-related indices and mortality was observed in those younger than 65 years, whereas these associations were attenuated to some extent in those older than 65 years (Additional file 1: Figs. S7 and S8). The associations were attenuated in those with LDL-C < 2.6 mmol/L, particularly as the TyG index even lost statistical significance (Additional file 1: Figs. S9 and S10). A weakened association pattern was also observed in those taking statins but not in those not taking statins (Additional file 1: Figs. S11 and S12). No substantial changes in the established associations were found when men and women were analyzed separately (Additional file 1: Figs. S13 and S14). Results were comparable to those of the main analysis after additional adjustment for education level, marital status, and poverty income ratio (Additional file 1: Fig. S15). The cumulative incidence of all-cause mortality and cause-specific mortality were incrementally increasing in the higher quartile level of the TyG-related indices group (Additional file 1: Fig. S16). The ROC curves showed that the predictive performance of the TyG-WHtR index for all-cause and cause-specific mortality was higher than that of the TyG index alone, whereas the predictive performance of the TyG-WC index was comparable to that of the TyG index (Additional file 1: Fig. S17). Finally, Pearson’s correlations between HOMA-IR and TyG-related indices were moderate, and the correlations between HOMA-IR and TyG-WHtR or TyG-WC indices were stronger than those with the TyG index alone (Additional file 1: Fig. S18).
Discussion
Results from this nationally representative longitudinal cohort study showed that elevated TyG, TyG-WHtR, and TyG-WC indices, either as continuous or categorical variables, were associated with all-cause and cause-specific mortality in the general population, independent of traditional clinical risk factors. These associations were more pronounced in younger individuals, those with high LDL-C levels, and those not taking statins. The TyG, TyG-WHtR, and TyG-WC indices showed independent dose-response associations with deaths from diabetes, heart disease, and cancer. The TyG index in combination with other anthropometric indicators reflecting central adiposity also maintained a prominent role in predicting mortality risk. These findings suggest that TyG index and TyG-derived indices can be used as simple and effective surrogate indicators for stratifying mortality risk in the general population to facilitate individualized surveillance and management.
Previous studies
Data from observational cohort studies suggest that the TyG index may serve as a valuable indicator for the early identification of individuals at high risk for cardiovascular events, including myocardial infarction and stroke [18, 19]. The predictive properties of the TyG index for atrial fibrillation, acute decompensated heart failure, premature stroke, acute kidney injury, and even female infertility have also been reported [20, 21]. In addition, cumulative exposure, variability, and dynamic trajectory of the TyG index have been significantly associated with higher risk of cardiovascular disease events [22–24]. Further evidence supports the predictive performance of the TyG index for major adverse outcomes in various clinical settings, including acute or chronic coronary syndromes, critical illness, and heart failure with different ejection fraction phenotypes [25–28]. However, the potential relationship between the TyG index and all-cause and cause-specific mortality has not been extensively studied, and there are inconsistencies among available data. An analysis of critically ill patients with coronary artery disease showed a linear positive association between the TyG index and in-hospital mortality [27]. In an observational cohort of cardiovascular patients with diabetes or pre-diabetes, the TyG index showed a U-shaped association with all-cause and cardiovascular mortality [29]. In contrast, the prospective PURE study found no clear association between TyG index and all-cause and non-cardiovascular mortality, whereas a statistically significant association was found for cardiovascular mortality [29]. A meta-analysis found no clear association between the TyG index and all-cause mortality. In fact, of the four studies they included, two found a significant association, while the other two did not [30]. These studies were conducted in specific clinical scenarios or populations, with small sample sizes or insufficient follow-up time, which may have inherently weakened their statistical power. In addition, heterogeneity in population, geographic location, and economic status may have contributed to these discrepant results. Given the conflicting evidence, further comprehensive validation of the association between the TyG index and risk of death in the general population is warranted.
Positive association between TyG-related indices and mortality
Our study included 27,642 individuals from a nationally representative cohort with a median follow-up of 10 years. We found a nonlinear J-shaped relationship between the TyG index and all-cause, cardiovascular and non-cardiovascular mortality, with risk inflection points ranging from 9.07 to 9.43. The current results are broadly consistent with previous findings that the association pattern between TyG index and all-cause and cardiovascular mortality was exhibited as J-shaped with statistically significant inflection points of 9.45 and 9.52 in hypertensive patients [31], as U-shaped with inflection points of 9.16 and 9.18 in diabetic patients [32], and as positively J-shaped with cut-offs of 9.36 and for 9.52 in the general population [33]. These findings underscore the importance of controlling the TyG index within a reasonable range to improve survival expectations, as the risk of death increases significantly once the threshold is exceeded. We observed a positive J-shaped association between the TyG-WHtR and TyG-WC indices and all-cause and cause-specific mortality in the general population, with risk inflection points ranging from 3.55 to 4.25 for the TyG-WHtR index, and from 783 to 886 for the TyG-WC index. Interestingly, the TyG-WHtR and TyG-WC indices appeared to have stronger statistical relevance compared to the native TyG index, especially the former. A recent study has confirmed the predictive power of TyG-related indices for all-cause, cardiovascular and diabetes mortality in metabolic syndrome populations [34], and we have further extended these findings to the general population, which allows for more extrapolation, and the study endpoints also included non-cardiovascular mortality (not limited to diabetes mortality). To our knowledge, this is the first study to demonstrate an independent prognostic role for these TyG-related indices in the general population. In addition, the TyG index in combination with other anthropometric indicators reflecting central adiposity steadily maintained such a monotonic positive association. However, this statistical association did not exist when the TyG index was combined with anthropometric measures reflecting overall adiposity. Abdominal or visceral obesity is a recognized risk factor for metabolic abnormalities, which leads to IR, dyslipidemia, and systemic inflammation. Individuals who are overweight or obese are more likely to develop IR, suggesting early impairment of glucose metabolism [35]. Given the strong relationship between IR and obesity and the fact that anthropometric measures reflecting abdominal obesity with WHtR and WC are more predictive of adverse cardiometabolic outcomes than traditional measures reflecting overall adiposity with BMI [14], the current findings are biologically plausible.
We assessed heterogeneity across subgroups employing an interaction term, which revealed significant effect modification between TyG-related indices and outcomes across several strata, including age, LDL-C level, and statin use. Those younger than 65 years were found to have a higher risk of mortality as TyG-related indices increased. These results are in line with previous findings that the TyG index is more predictive of the incidence of coronary artery disease, ischemic stroke, and all-cause and cardiovascular mortality in young adults [36, 37]. There are several possible explanations. First, the elderly may present with a variety of metabolic disorders, such as hyperglycemia, dyslipidemia, and hypertension, which are closely associated with clinical prognosis. Due to the existence of multiple metabolic disturbances, it is possible that the TyG index may not be the sole or the strongest predictor. Second, the elderly may be using multiple medications, including lipid-lowering and hypoglycemic drugs, potentially influencing the level of the TyG index and thus its predictive performance. Numerous randomized controlled trials have shown that LDL-C lowering and statin use have unequivocal clinical benefits for primary and secondary prevention in patients with ASCVD [38]. We found that the association between TyG-related indices and mortality was attenuated in individuals with LDL-C levels below 2.6 mmol/L and statin use, suggesting that control of LDL-C to low levels and statin use may partially offset the adverse effects of an elevated TyG index. Under physiological conditions, insulin degrades apolipoprotein B-containing lipoproteins by activating PI3K, thereby reducing the synthesis of LDL, very-low-density lipoprotein, and other atherogenic lipids. Conversely, insulin resistance inhibits this degradation, leading to increased production of LDL-C and other atherogenic cholesterol components, contributing to systemic lipid disorders [39]. This close interplay between IR and lipid metabolism may provide a potential explanation for the current findings that control of LDL-C to low levels and the prescription of statins appear to be protective for long-term survival when IR is triggered.
In the analyses for more detailed subcategories of cardiovascular mortality, the significant association between TyG-related indices and death from heart disease can be mechanistically explained by IR-related cardiovascular damage. The association of TyG-related indices with non-cardiovascular mortality was nominally attributed to increased mortality from all other mortality subcategories except Alzheimer’s disease and nephropathy, and especially diabetes mellitus. In addition, we found a significant association between TyG indices and cancer mortality. Previous research has shown that IR-induced hyperinsulinemia promotes the production of inflammatory cytokines as well as the activation of insulin-like growth factor signaling pathways, which may increase tumor aggressiveness and promotes its malignant phenotype [40]. A statistically significant association has also been found between the TyG-WHtR and TyG-WC indices and death from respiratory disease, which may be explained by the lung function impairment and inflammatory activation associated with IR [41].
Potential explanations
The exact mechanism by which TyG-related indices are associated with mortality is unknown, and IR may play a key role through down-regulating the insulin signaling. IR is manifested by increased insulin vulnerability and decreased insulin bioavailability, which diminishes the capacity of insulin-targeted tissues to adequately dispose glucose at elevated plasma insulin concentrations, leading to impaired glucose homeostasis. IR is closely linked to a series of metabolic dysregulations, such as lipotoxic insulin signaling, hyperglycemia and dyslipidemia, and adipose tissue dysfunction. These metabolic disturbances are well established by epidemiological or genetic evidence as important causes of cardiovascular mortality and all-cause mortality [42, 43]. In addition, IR can induce other deleterious biological effects, including oxidative stress, low-grade inflammation, dysfunctional immune modulation, impaired endothelial function, and prothrombotic state, all of which contribute to vascular sclerosis and senescence and compromise multiple organ function [3, 4, 44]. Therefore, severe IR not only exhibits metabolic abnormalities, but also carries a high risk of ASCVD, hypertension, heart failure, renal and hepatic comorbidities, ultimately leading to premature death.
Clincal importance
The current findings have important clinical implications. First, individuals with high TyG-related indices appear to be at particularly high risk of death, suggesting the need for intensive surveillance and tailored interventions to minimize absolute risk. In terms of public health impact, detection of TyG-related indices in the general population allows for early identification of high-risk individuals and initiation of therapeutic interventions, which is particularly relevant for global health policy-making to reduce overall population mortality. Second, TyG-related indices are suitable for daily clinical practice because they are easily obtained from routine laboratory variables, do not require insulin quantification, and are not influenced by insulin treatment status. Third, the association between TyG-related indices and mortality varies by age, LDL-C level, and statin use, highlighting the necessity of stratified management for different risk groups. Finally, there is an even better predictive performance when the TyG index is combined with anthropometric measures, especially WHtR. Therefore, these TyG-derived indices deserve to be added to routine health screening programs and widely promoted in clinical applications. A potentially reasonable recommendation is that all adults should undergo at least one TyG index test to encourage early discovery of metabolic risk and to inform clinical decision making.
Strengths and limitations
Strengths of this study include a large-scale, prospective, population-based study design, long-term follow-up with few losses, and sufficient endpoint events to ensure statistical power. In addition, multiple sensitive analyses demonstrated the robustness of the main findings. Several limitations should be noted. First, because of the inherent limitations of observational study designs, the current results do not indicate causality. Although individuals with less than 2 years of follow-up were excluded and the estimates were similar, reverse causality could not be completely avoided. Second, despite our efforts to extensively adjust for potential confounders, residual confounders may still exist. Nevertheless, the statistical E-values for the associations between the highest quartiles of TyG-related indices and all-cause and cause-specific mortality ranged from 1.76 to 3.12, implying that the unmeasured confounders should have an association with the exposure (the highest quartiles) and outcome (mortality) comparable to these values to negate the current results (Additional file 1: Fig. S19). Third, anthropometric and blood indices were only collected at a single time point and may not capture the long-term exposure trajectory of TyG-related indices over time. Finally, analyses of cause-specific mortality subcategories are highly exploratory because the limited number of events does not allow for reliable statistical significance, and thus these results need to be replicated in future studies with larger sample sizes.
Conclusions
TyG-related indices were significantly associated with all-cause and cause-specific mortality in the general population. These associations were pronounced in younger individuals, those with high LDL-C levels, and those not taking statins.
Supplementary Information
Acknowledgements
The authors would like to express their sincere gratitude to the NCHS for making the NHANES databases publicly available for clinical research.
Abbreviations
- TyG
Triglyceride-glucose
- BMI
Body mass index
- WC
Waist circumference
- WHtR
Waist-to-height ratio
- BRI
Body roundness index
- BSI
Body shape index
- WWI
Weight-adjusted-waist index
- RFM
Relative fat mass
- CI
Conicity index
- IR
Insulin resistance
- NHANES
National health and nutrition examination survey
- NDI
National death index
- NCHS
National center for health statistics
- HIEC
Hyperinsulinaemic-euglycaemic clamp
- HOMA-IR
Homeostasis model assessment for insulin resistance
- QUICKI
Quantitative insulin sensitivity checking index
- TG
Triglyceride
- FBG
Fasting blood glucose
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- ASCVD
Atherosclerotic cardiovascular disease
- CHF
Congestive heart failure
- COPD
Chronic obstructive pulmonary disease
- PIR
Poverty income ratio
- VIF
Variance inflation factor
- HOMA-IR
Homeostasis model assessment of insulin resistance index
Author contributions
LH had full access to all the data and takes responsibility for the integrity of the data and the accuracy of the analysis. LS and AL contributed to the the conception and design of the study. FZ and ZW contributed to data acquisition, statistical analysis, and interpretation. LS contributed to the drafting of the manuscript. All authors were responsible for the critical revision of the manuscript for important content.
Funding
This work was supported by the National Key Research Program of China (2020YFC2008304) and the Key Projects of Logistics Scientific Research Project of Chinese PLA (22BJZ26).
Data availability
The datasets generated and analysed in this study are available in the NHANES repository (https://www.cdc.gov/nchs/nhanes/index.htm).
Declarations
Ethics approval and consent to participate
The NHANES study protocol was approved by the Ethics Review Board of the National Center for Health Statistics. Written informed consent was obtained from all participants.
Consent for publication
All authors have consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shan Li and Li An have contributed equally to this work.
References
- 1.World health statistics. 2024: monitoring health for the SDGs, sustainable development goals. [https://www.who.int/data/gho/publications/world-health-statistics. Accessed 21 May 2024.].
- 2.Mastrototaro L, Roden M. Insulin resistance and insulin sensitizing agents. Metab Clin Exp. 2021;125:154892. 10.1016/j.metabol.2021.154892 [DOI] [PubMed] [Google Scholar]
- 3.Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med: J Br Diabet Assoc. 2003;20(4):255–68. 10.1046/j.1464-5491.2003.00869.x [DOI] [PubMed] [Google Scholar]
- 4.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144–53. 10.1038/nrendo.2015.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloomgarden ZT. Measures of insulin sensitivity. Clin Lab Med. 2006;26(3):611–33 vi. 10.1016/j.cll.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 6.Tura A, Chemello G, Szendroedi J, Göbl C, Færch K, Vrbíková J, Pacini G, Ferrannini E, Roden M. Prediction of clamp-derived insulin sensitivity from the oral glucose insulin sensitivity index. Diabetologia. 2018;61(5):1135–41. 10.1007/s00125-018-4568-4 [DOI] [PubMed] [Google Scholar]
- 7.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metabolism. 2008;294(1):E15-26. 10.1152/ajpendo.00645.2007 [DOI] [PubMed] [Google Scholar]
- 8.Park HM, Lee HS, Lee YJ, Lee JH. The triglyceride-glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract. 2021;180:109042. 10.1016/j.diabres.2021.109042 [DOI] [PubMed] [Google Scholar]
- 9.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20(1):76. 10.1186/s12933-021-01268-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalaji A, Behnoush AH, Khanmohammadi S, Ghanbari Mardasi K, Sharifkashani S, Sahebkar A, Vinciguerra C, Cannavo A. Triglyceride-glucose index and heart failure: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22(1):244. 10.1186/s12933-023-01973-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Gui J, Liu H, Guo LL, Li J, Lei Y, Li X, Sun L, Yang L, Yuan T, et al. Predicting metabolic syndrome by obesity- and lipid-related indices in mid-aged and elderly Chinese: a population-based cross-sectional study. Front Endocrinol. 2023;14:1201132. 10.3389/fendo.2023.1201132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1):279. 10.1186/s12933-023-02030-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev: Off J Int Assoc Study Obes. 2012;13(3):275–86. 10.1111/j.1467-789X.2011.00952.x [DOI] [PubMed] [Google Scholar]
- 14.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Reviews Endocrinol. 2020;16(3):177–89. 10.1038/s41574-019-0310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X, Liu L, Ming Z, Tao X, Li Y. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc Diabetol. 2024;23(1):8. 10.1186/s12933-023-02115-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Sun M, Yang Y, Yao N, Yan S, Wang L, Hu W, Guo R, Wang Y, Li B. Predictive effect of triglyceride glucose-related parameters, obesity indices, and lipid ratios for diabetes in a Chinese population: a prospective cohort study. Front Endocrinol. 2022;13:862919. 10.3389/fendo.2022.862919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel CJ, Pho N, McDuffie M, Easton-Marks J, Kothari C, Kohane IS, Avillach P. A database of human exposomes and phenomes from the US national health and nutrition examination survey. Sci data. 2016;3:160096. 10.1038/sdata.2016.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361. 10.1186/s12916-020-01824-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao Y, Hu H, Li Q, Cao C, Liu D, Han Y. Link between triglyceride-glucose-body mass index and future stroke risk in middle-aged and elderly Chinese: a nationwide prospective cohort study. Cardiovasc Diabetol. 2024;23(1):81. 10.1186/s12933-024-02165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang R, Wang Z, Chen J, Bao X, Xu N, Guo S, Gu R, Wang W, Wei Z, Wang L. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 2022;21(1):88. 10.1186/s12933-022-01507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W, Zhao H, Han X, Liu J, Li H, Sun J, Xing A, Chen S, Wu S, Wu Y. Relationship between early-onset stroke and triglyceride-glucose index among young Chinese adults. Lipids Health Dis. 2023;22(1):3. 10.1186/s12944-023-01773-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao JW, Hao QY, Gao M, Zhang K, Li XZ, Wang JF, Vuitton DA, Zhang SL, Liu PM. Triglyceride-glucose index in the development of peripheral artery disease: findings from the atherosclerosis risk in communities (ARIC) Study. Cardiovasc Diabetol. 2021;20(1):126. 10.1186/s12933-021-01319-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Zuo Y, Qian F, Chen S, Tian X, Wang P, Li X, Guo X, Wu S, Wang A. Triglyceride-glucose index variability and incident cardiovascular disease: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):105. 10.1186/s12933-022-01541-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Huang R, Lin Y, Guo Y, Xiong Z, Zhong X, Ye X, Li M, Zhuang X, Liao X. High triglyceride-glucose index in young adulthood is associated with incident cardiovascular disease and mortality in later life: insight from the CARDIA study. Cardiovasc Diabetol. 2022;21(1):155. 10.1186/s12933-022-01593-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Yang J, Wang K, Niu J, Liu Y, Ge H. Association between the triglyceride-glucose index and in-hospital major adverse cardiovascular events in patients with acute coronary syndrome: results from the improving care for cardiovascular disease in China (CCC)-acute coronary syndrome project. Cardiovasc Diabetol. 2024;23(1):170. 10.1186/s12933-024-02270-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Liu L, Chen H, Li S, Wan M, Mohammed AQ, Xu B, Yin G, Lv X, Shi T, et al. Association between the triglyceride-glucose index and the presence and prognosis of coronary microvascular dysfunction in patients with chronic coronary syndrome. Cardiovasc Diabetol. 2023;22(1):113. 10.1186/s12933-023-01846-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R, Shi S, Chen W, Wang Y, Lin X, Zhao Y, Liao L, Guo Q, Zhang X, Li W, et al. Independent effects of the triglyceride-glucose index on all-cause mortality in critically ill patients with coronary heart disease: analysis of the MIMIC-III database. Cardiovasc Diabetol. 2023;22(1):10. 10.1186/s12933-023-01737-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Yang J, Tang H, Guo Z, Dong W, Wang Y, Meng X, Zhang K, Wang W, Shao C, et al. High triglyceride-glucose (TyG) index is associated with poor prognosis of heart failure with preserved ejection fraction. Cardiovasc Diabetol. 2023;22(1):263. 10.1186/s12933-023-02001-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Jaramillo P, Gomez-Arbelaez D, Martinez-Bello D, Abat MEM, Alhabib KF, Avezum Á, Barbarash O, Chifamba J, Diaz ML, Gulec S, et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 2023;4(1):e23-33. 10.1016/S2666-7568(22)00247-1 [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, Ma J, Zhao Y, Zhu W, Wang J. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124. 10.1186/s12933-022-01546-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou D, Liu XC, Kenneth L, Huang YQ, Feng YQ. A Non-linear association of triglyceride glycemic index with cardiovascular and all-cause mortality among patients with hypertension. Front Cardiovasc Med. 2021;8:778038. 10.3389/fcvm.2021.778038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C, Liang D, Xiao K, Xie L. Association between the triglyceride-glucose index and all-cause and CVD mortality in the young population with diabetes. Cardiovasc Diabetol. 2024;23(1):171. 10.1186/s12933-024-02269-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med. 2020;7:628109. 10.3389/fcvm.2020.628109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei X, Min Y, Song G, Ye X, Liu L. Association between triglyceride-glucose related indices with the all-cause and cause-specific mortality among the population with metabolic syndrome. Cardiovasc Diabetol. 2024;23(1):134. 10.1186/s12933-024-02215-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis: NMCD. 2007;17(4):319–26. 10.1016/j.numecd.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 36.Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: tehran lipid and glucose study. Cardiovasc Diabetol. 2020;19(1):155. 10.1186/s12933-020-01121-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Wu K, Lin Y, Huang M, Xie S. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc Diabetol. 2023;22(1):320. 10.1186/s12933-023-02054-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 39.Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–99. 10.1007/s00125-015-3525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Ruan S, Wu Z, Yan Q, Chen Y, Cui J, Zhang Z, Huang S, Hou B, Zhang C. Prognostic significance of glucose-lipid metabolic index in pancreatic cancer patients with diabetes mellitus. Cancer Med. 2024;13(6):e7108. 10.1002/cam4.7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baffi CW, Wood L, Winnica D, Strollo PJ Jr, Gladwin MT, Que LG, Holguin F. Metabolic syndrome and the lung. Chest. 2016;149(6):1525–34. 10.1016/j.chest.2015.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet (London England). 2014;384(9943):626–35. 10.1016/S0140-6736(14)61177-6 [DOI] [PubMed] [Google Scholar]
- 43.Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, Yang Y, Hu Y, Huang Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ (Clin Res Ed). 2020;370:m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. 2017;113(4):389–98. 10.1093/cvr/cvx012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed in this study are available in the NHANES repository (https://www.cdc.gov/nchs/nhanes/index.htm).