Abstract
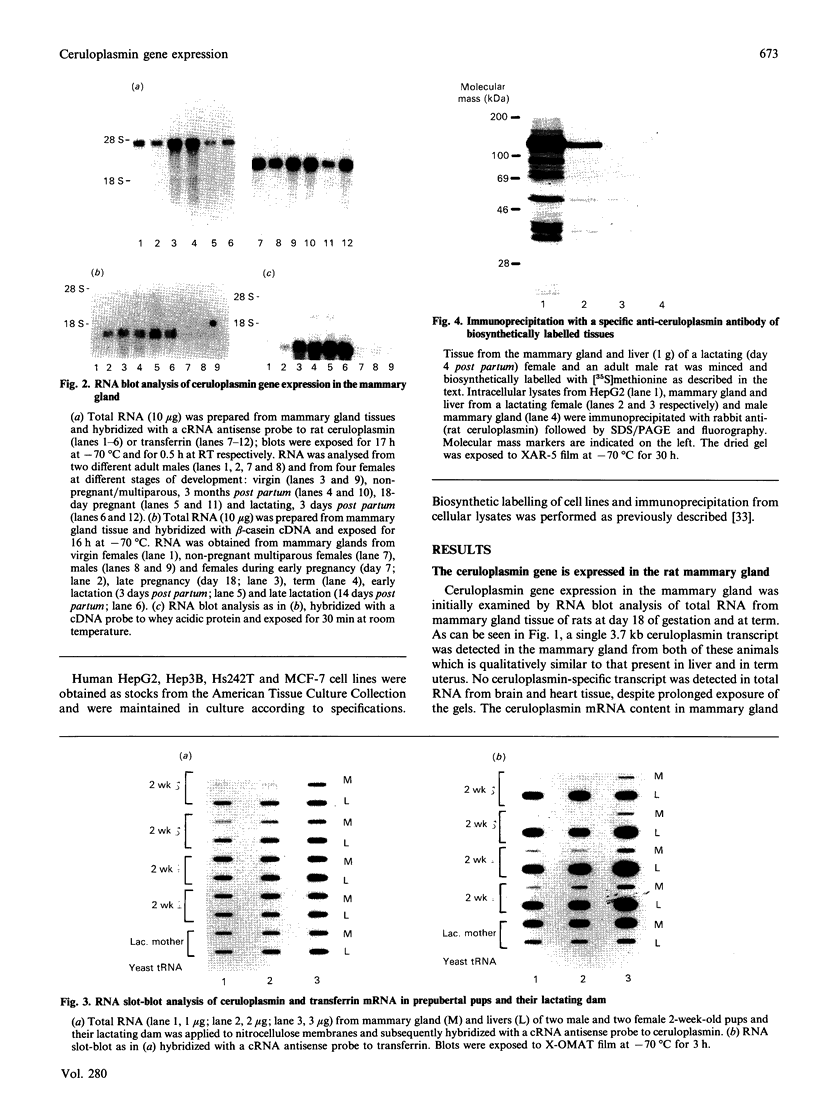
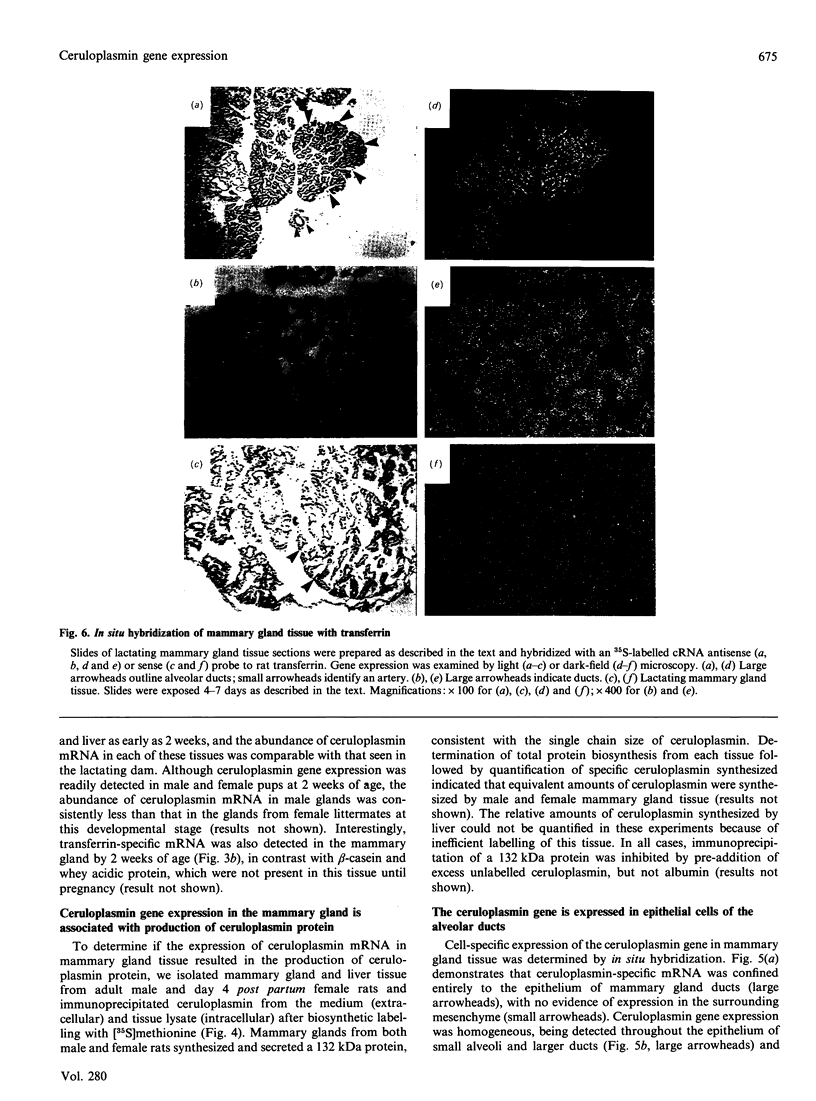
Using a ceruloplasmin cDNA clone in RNA blot analysis, a single 3.7 kb ceruloplasmin-specific transcript was detected in rat mammary gland tissue from pregnant and lactating animals. Ceruloplasmin gene expression in the mammary gland was tissue-specific, with no evidence of expression in brain, heart or other extrahepatic tissues. Ceruloplasmin mRNA was also detected in mammary gland tissue from male, virgin female and non-pregnant/multiparous animals, and the abundance of ceruloplasmin-specific transcripts in virgin female rats was independent of their stage of oestrus. In virgin female mammary gland the content of ceruloplasmin mRNA was 20% of that in hepatic tissue from these animals and approx. 2-3-fold greater than that found in mammary gland tissue of pregnant or lactating animals. Development studies revealed ceruloplasmin gene expression in male and female mammary gland by only 2 weeks of age, prior to the onset of puberty. Biosynthetic studies indicated that the ceruloplasmin mRNA in mammary gland tissue was translated into a 132 kDa protein qualitatively similar to that synthesized in liver. By in situ hybridization, ceruloplasmin gene expression was localized to the epithelium lining the mammary gland alveolar ducts, without evidence of expression in the surrounding mesenchyme. Ceruloplasmin gene expression was also detected in a human breast adenocarcinoma cell line and in biopsy tissue from women with invasive ductal carcinoma. Taken together, these data indicate that the mammary gland is a prominent site of extrahepatic ceruloplasmin gene expression and add to the evidence that ceruloplasmin biosynthesis is associated with growth and differentiation in non-hepatic tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldred A. R., Grimes A., Schreiber G., Mercer J. F. Rat ceruloplasmin. Molecular cloning and gene expression in liver, choroid plexus, yolk sac, placenta, and testis. J Biol Chem. 1987 Feb 25;262(6):2875–2878. [PubMed] [Google Scholar]
- Bayna E. M., Rosen J. M. Tissue-specific, high level expression of the rat whey acidic protein gene in transgenic mice. Nucleic Acids Res. 1990 May 25;18(10):2977–2985. doi: 10.1093/nar/18.10.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. L., Zeigler M. E., Wicha M. S. Regulation of rat mammary gene expression by extracellular matrix components. Exp Cell Res. 1987 Dec;173(2):322–340. doi: 10.1016/0014-4827(87)90274-6. [DOI] [PubMed] [Google Scholar]
- Chen L. H., Bissell M. J. Transferrin mRNA level in the mouse mammary gland is regulated by pregnancy and extracellular matrix. J Biol Chem. 1987 Dec 25;262(36):17247–17250. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cousins R. J. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985 Apr;65(2):238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fleming R. E., Gitlin J. D. Primary structure of rat ceruloplasmin and analysis of tissue-specific gene expression during development. J Biol Chem. 1990 May 5;265(13):7701–7707. [PubMed] [Google Scholar]
- Fleming R. E., Whitman I. P., Gitlin J. D. Induction of ceruloplasmin gene expression in rat lung during inflammation and hyperoxia. Am J Physiol. 1991 Feb;260(2 Pt 1):L68–L74. doi: 10.1152/ajplung.1991.260.2.L68. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Frieden E. Perspectives on copper biochemistry. Clin Physiol Biochem. 1986;4(1):11–19. [PubMed] [Google Scholar]
- Gitlin J. D., Gitlin J. I., Gitlin D. Localizing of C-reactive protein in synovium of patients with rheumatoid arthritis. Arthritis Rheum. 1977 Nov-Dec;20(8):1491–1499. doi: 10.1002/art.1780200808. [DOI] [PubMed] [Google Scholar]
- Gitlin J. D., Gitlin J. I., Gitlin D. Selective transfer of plasma proteins across mammary gland in lactating mouse. Am J Physiol. 1976 Jun;230(6):1594–1602. doi: 10.1152/ajplegacy.1976.230.6.1594. [DOI] [PubMed] [Google Scholar]
- Gitlin J. D. Transcriptional regulation of ceruloplasmin gene expression during inflammation. J Biol Chem. 1988 May 5;263(13):6281–6287. [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Grigor M. R., McDonald F. J., Latta N., Richardson C. L., Tate W. P. Transferrin-gene expression in the rat mammary gland. Independence of maternal iron status. Biochem J. 1990 May 1;267(3):815–819. doi: 10.1042/bj2670815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Biosynthesis and assembly of the alpha and beta subunits of Mac-1, a macrophage glycoprotein associated with complement receptor function. J Biol Chem. 1983 Mar 10;258(5):2766–2769. [PubMed] [Google Scholar]
- Kingston I. B., Kingston B. L., Putnam F. W. Chemical evidence that proteolytic cleavage causes the heterogeneity present in human ceruloplasmin preparations. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5377–5381. doi: 10.1073/pnas.74.12.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussis D., Eiferman F., van de Rijn P., Gorin M. B., Ingram R. S., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. II. The structures of the alpha-fetoprotein and albumin genes in the mouse. J Biol Chem. 1981 Feb 25;256(4):1960–1967. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. F., Atiee S. H., Rosen J. M. Differential regulation of rat beta-casein-chloramphenicol acetyltransferase fusion gene expression in transgenic mice. Mol Cell Biol. 1989 Feb;9(2):560–565. doi: 10.1128/mcb.9.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkilä H., Gitlin J. D., Muller-Eberhard U. Rat hemopexin. Molecular cloning, primary structural characterization, and analysis of gene expression. Biochemistry. 1991 Jan 22;30(3):823–829. doi: 10.1021/bi00217a036. [DOI] [PubMed] [Google Scholar]
- O'Malley K. L., Anhalt M. J., Martin B. M., Kelsoe J. R., Winfield S. L., Ginns E. I. Isolation and characterization of the human tyrosine hydroxylase gene: identification of 5' alternative splice sites responsible for multiple mRNAs. Biochemistry. 1987 Nov 3;26(22):6910–6914. doi: 10.1021/bi00396a007. [DOI] [PubMed] [Google Scholar]
- Raju K. S., Alessandri G., Ziche M., Gullino P. M. Ceruloplasmin, copper ions, and angiogenesis. J Natl Cancer Inst. 1982 Nov;69(5):1183–1188. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokyszyn V. M., Miller D. M., Reif D. W., Aust S. D. Inhibition of superoxide and ferritin-dependent lipid peroxidation by ceruloplasmin. J Biol Chem. 1989 Jan 5;264(1):21–26. [PubMed] [Google Scholar]
- Sato M., Gitlin J. D. Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J Biol Chem. 1991 Mar 15;266(8):5128–5134. [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Sertoli cells synthesize and secrete a ceruloplasmin-like protein. Biol Reprod. 1983 Jun;28(5):1225–1229. doi: 10.1095/biolreprod28.5.1225. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Ortel T. L., Putnam F. W. Single-chain structure of human ceruloplasmin: the complete amino acid sequence of the whole molecule. Proc Natl Acad Sci U S A. 1984 Jan;81(2):390–394. doi: 10.1073/pnas.81.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao T., Owen C. A., Jr Copper metabolism in pregnant and pospartum rat and pups. Am J Physiol. 1977 Feb;232(2):E172–E179. doi: 10.1152/ajpendo.1977.232.2.E172. [DOI] [PubMed] [Google Scholar]
- Thomas T., Schreiber G. The expression of genes coding for positive acute-phase proteins in the reproductive tract of the female rat. High levels of ceruloplasmin mRNA in the uterus. FEBS Lett. 1989 Jan 30;243(2):381–384. doi: 10.1016/0014-5793(89)80166-8. [DOI] [PubMed] [Google Scholar]
- Walker F. J., Fay P. J. Characterization of an interaction between protein C and ceruloplasmin. J Biol Chem. 1990 Feb 5;265(4):1834–1836. [PubMed] [Google Scholar]
- Weiner A. L., Cousins R. J. Hormonally produced changes in caeruloplasmin synthesis and secretion in primary cultured rat hepatocytes. Relationship to hepatic copper metabolism. Biochem J. 1983 May 15;212(2):297–304. doi: 10.1042/bj2120297. [DOI] [PMC free article] [PubMed] [Google Scholar]