Abstract
Purpose
Retinal neovascularization is a significant feature of advanced age-related macular degeneration (AMD) and a major cause of blindness in patients with AMD. However, the underlying mechanism of this pathological neovascularization remains unknown. Iron metabolism has been implicated in various biological processes. This study was conducted to investigate the effects of iron metabolism on retinal neovascularization in neovascular AMD (nAMD).
Methods
C57BL/6J and very low-density lipoprotein receptor (VLDLR) knockout (Vldlr−/−) mice, a murine model of nAMD, were used in this study. Bulk-RNA sequencing was used to identify differentially expressed genes. Western blot analysis was performed to test the expression of proteins. Iron chelator deferiprone (DFP) was administrated to the mice by oral gavage. Fundus fluorescein angiography was used to evaluate retinal vascular leakage. Immunofluorescence staining was used to detect macrophages and iron-related proteins.
Results
RNA sequencing (RNA-seq) results showed altered transferrin expression in the retina and RPE of Vldlr−/− mice. Disrupted iron homeostasis was observed in the retina and RPE of Vldlr−/− mice. DFP mitigated iron overload and significantly reduced retinal neovascularization and vascular leakage. In addition, DFP suppressed the inflammation in Vldlr−/− retinas. The reduced signals of macrophages were observed at sites of neovascularization in the retina and RPE of Vldlr−/− mice after DFP treatment. Further, the IL-6/JAK2/STAT3 signaling pathway was activated in the retina and RPE of Vldlr−/− mice and reversed by DFP treatment.
Conclusions
Disrupted iron metabolism may contribute to retinal neovascularization in nAMD. Restoring iron homeostasis by DFP could be a potential therapeutic approach for nAMD.
Keywords: age-related macular degeneration (AMD), deferiprone (DFP), iron metabolism; retinal neovascularization, Vldlr−/− mice, JAK2-STAT3 pathway
Age-related macular degeneration (AMD) is a leading cause of vision loss in the elderly in developed countries, affecting 196 million people worldwide in 2020. These numbers are expected to rise to 288 million by 2040.1 Neovascular AMD (nAMD) is an advanced stage of AMD characterized by neovascularization, also known as wet AMD. The mechanism of nAMD is still unclear, and the clinical treatments for nAMD are nonspecific and limited. Recently, intravitreally administered anti-VEGF therapy has become the first-line treatment for nAMD. However, anti-VEGF therapy has many limitations, including the frequent need for intravitreal injections, retinal fibrosis, high cost, and reduced efficacy.2 Finding new avenues for therapeutic intervention is critical to improving the prognosis of nAMD.
Iron is an essential element for the development and survival of virtually all living organisms, playing a pivotal role in cellular functions and energy metabolism.3 Iron overload has been implicated in multiple diseases, such as Parkinson's disease, amyotrophic lateral sclerosis, and Alzheimer's disease.4–6 Abnormal iron accumulation has also been found to contribute to the pathological process of several ophthalmic diseases.7–10 For example, pathologically high intraocular pressure leads to excessive iron accumulation in the murine retina.7 In patients with retinal detachment, toxic iron accumulates in the vitreous, photoreceptor layer, and RPE, which is associated with poor visual recovery after surgical reattachment.8 Increased iron levels have also been reported in the aqueous humor, RPE, and Bruch’s membrane of patients with AMD.9,10 In addition, mice deficient in the ferroxidase ceruloplasmin (Cp) and hephaestin (Heph) develop age-dependent iron overload in the RPE and retina, and display age-dependent RPE hypertrophy, photoreceptor degeneration, and subretinal neovascularization.11 Therefore, these studies suggest that disrupted iron homeostasis is associated with AMD. However, whether iron metabolism is involved in retinal neovascularization in nAMD is unknown.
The suppression of iron overload has been shown to have beneficial effects.7,12–14 For example, chelating excess iron can inhibit the loss of retinal ganglion cells caused by pathologically high intraocular pressure, and attenuate the visual impairment triggered by retinal ischemia-reperfusion injuries.7,12 It was reported that iron accumulated in the retina of rd10 mutant mice and the overexpression or systemic administration of human transferrin, a potent iron chelator, could reduce photoreceptor cell death in rd10 mice.13 Picard et al. reported that intravitreal injection of transferrin decreased iron content and oxidative stress and protected photoreceptors against cell death in a murine model of light-induced retinal degeneration.14 Because the iron levels are elevated in the eyes of patients with AMD, we hypothesize that iron plays a role in the development of retinal neovascularization and restoring iron homeostasis may have beneficial effects on nAMD.
In this study, we identified that very low-density lipoprotein receptor (Vldlr−/−) knockout mice, an nAMD animal model, have elevated iron levels, which may mimic the elevated iron condition in patients with nAMD. In addition, the iron chelator deferiprone (DFP) restores iron homeostasis and inhibits retinal neovascularization. A possible mechanism for the action of DFP was also investigated. Our results indicated that iron levels and distribution can influence retinal neovascularization, and the reduction of iron overload by iron chelator may provide new insights for the treatment of nAMD.
Materials and Methods
Animals
Wild-type (WT) mice C57BL/6J were obtained from the Laboratory Animal Center of Xiamen University (Xiamen, China). VLDLR knockout (Vldlr−/−) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Littermate Vldlr−/− pups were randomly divided into groups and administered DFP (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 100 mg/kg or saline through intragastric gavage once daily from p12 to p21. The experimental procedures were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and approved by the Xiamen University Experimental Animal Ethics Committee.
Western Blot Analysis
The Western blot analysis was performed as previously reported.15 Briefly, total protein was extracted using radio immunoprecipitation assay (RIPA) lysis buffer. Protein concentration was determined using the Pierce BCA protein assay kit (Thermo Fisher Scientific, Wilmington, DE, USA). Then, 10 µg of protein were electrophoresed onto an SDS-PAGE gel and transferred onto a polyvinylidene difluoride (PVDF) membrane (Roche, Basel, Switzerland). The antibodies used in Western blot analysis are shown in Table 1. All the RPE samplers mentioned in this manuscript were RPE/choroid complex.
Table 1.
Antibodies for Western Blot
| Primary Antibody | Brand | Catalog No. | Dilution |
|---|---|---|---|
| Transferrin | Abcam | ab82411 | 1:1000 |
| Transferrin receptor | Abcam | ab84036 | 1:50 |
| Ferritin | Abcam | ab75973 | 1:1000 |
| Ferritin heavy chain | Abcam | ab65080 | 1:1000 |
| Glial fibrillary acidic protein | Sigma-Aldrich | G3893 | 1:1000 |
| Vascular endothelial growth factor | Santa Cruz | SC-7269 | 1:2000 |
| Interleukin-6 | Abcam | ab7737 | 1:1000 |
| Janus kinase 2 | Cell Signaling Technology | 3230 | 1:1000 |
| Phospho-Janus kinase 2 | Cell Signaling Technology | 3776 | 1:1000 |
| Signal transducer and activator of transcription 3 | Cell Signaling Technology | 4904 | 1:2000 |
| Phospho-signal transducer and activator of transcription 3 | Cell Signaling Technology | 9145 | 1:2000 |
| Glyceraldehyde-3-phosphate dehydrogenase | Cell Signaling Technology | 2118 | 1:5000 |
| β-actin-HRP | Sigma-Aldrich | A3854 | 1:5000 |
| Goat anti-rabbit HRP | Abcam | ab205718 | 1:2000 |
| Goat anti-mouse HRP | Abcam | ab6789 | 1:2000 |
Total RNA Extraction and RNA Sequencing
Total RNA was extracted using RNAiso Plus (TaKaRa Bio, Inc., Shiga, Japan) following the manufacturer’s protocol. Four individual retinas or RPE were pooled as one sample. Three or four samplers in each group were subjected to further tests. RNA sequencing (RNA-seq) analysis was conducted by Beijing Genomics Institute (BGI, Shenzhen, China). The sequencing was performed using the BGISEQ-500 platform. Heatmaps, Gene Ontology (GO) classification, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, and protein-protein interaction (PPI) networks were performed on the BGI Dr. Tom platform.16
Quantitative Real-Time Polymerase Chain Reaction
The total RNA was reverse transcribed into cDNA using a PrimeScript RT Master Mix (TaKaRa). Quantitative RT-PCR (qRT-PCR) was conducted using the Hieff qPCR SYBR Green Master Mix (Yeasen, Shanghai, China) according to the manufacturer’s instructions. The sequences of primers are included in Table 2. The mRNA expression was normalized to that of β-actin and analyzed according to the comparative cycle threshold method.17
Table 2.
Primer Sequences for Quantitative Real-Time PCR
| Gene Name | Primer Sequences (5′-3′) |
|---|---|
| β-actin | Forward: TGAGAGGCAAATCGTGCGTGACAT |
| Reverse: ACCGCTCGTTGCCAATAGTGATGA | |
| Interleukin-1β | Forward: GATCCACACTCTCCAGCTGCA |
| Reverse: CAACCAACAAGTGATATTCTCCATG | |
| Vascular cell adhesion molecule-1 | Forward: CCCAGGTGGAGGTCTACTCA |
| Reverse: CAGGATTTTGGGAGCTGGTA | |
| Tumor necrosis factor-α | Forward: GCCTCTTCTCATTCCTGCTTG |
| Reverse: CTGATGAGAGGGAGGCCATT | |
| Adhesion G protein-coupled receptor E1 | Forward: CTTTGGCTATGGGCTTCCAGTC |
| Reverse: GCAAGGAGGACAGAGTTTATCGTG |
Immunofluorescence Staining
Intracardiac perfusions with PBS following with 4% paraformaldehyde (PFA) were performed on all the mice after euthanasia. Immunofluorescence staining was conducted as described previously.18 Briefly, cryosections (8 µm thickness) were permeabilized with 0.2% Triton X-100 for 20 minutes and blocked with 2% BSA in PBS for 1 hour at room temperature (RT). The sections were incubated with primary antibodies overnight at 4°C. The negative controls (without any primary antibody but with a corresponding secondary antibody) were conducted each time to identify nonspecific binding from the secondary antibody. All the immunofluorescent images of negative controls were presented in Supplementary Figure S1. After washing with PBS, the sections were incubated with the secondary antibodies for 1 hour at RT. The samples were mounted and analyzed with a confocal laser scanning microscope (Zeiss, Braunschweig, Germany; LSM 880) by ZEN imaging software. The antibodies used in immunostaining are shown in Table 3.
Table 3.
Antibodies for Immunofluorescence
| Antibody | Brand | Catalog No. | Dilution |
|---|---|---|---|
| Transferrin | Abcam | ab82411 | 1:50 |
| Ferritin heavy chain | Abcam | ab65080 | 1:200 |
| F4/80 | Abcam | ab6640 | 1:200 |
| CD31 | R&D Systems | AF3628 | 1:50 |
| Alexa Fluor 594-conjugated goat anti-rabbit | Abcam | ab150080 | 1:300 |
| Alexa Fluor 488-conjugated goat anti-rabbit | Abcam | ab150077 | 1:300 |
| Alexa Fluor 594-conjugated donkey anti-rabbit | Abcam | ab150076 | 1:300 |
| Alexa Fluor 488-conjugated donkey anti-goat | Abcam | ab150129 | 1:300 |
| Alexa Fluor 594-conjugated goat anti-rat | Abcam | ab150160 | 1:300 |
| Alexa Fluor 488-conjugated goat anti-rat | Abcam | ab150157 | 1:300 |
Whole Mount Staining of Retina and RPE/Choroid Complex
The eyeballs were enucleated and fixed in 4% PFA for 1 hour at RT. The lectin staining of retinas was performed according to a previously published protocol.19 The RPE/choroid complex staining was performed as follows: the RPE/choroid complex was blocked in PBS with 5% goat serum and 1% Triton X-100 for 2 hours at RT. After washing with PBS, the RPE/choroid complex was incubated with F4/80 antibody and GS-IB4 overnight at 4°C. After washing with PBS, the complex was incubated with Alexa Fluor 488-conjugated IgG and DAPI overnight at 4°C. The retina or RPE/choroid complex was mounted, and images were obtained using a Zeiss LSM 880 confocal microscope. IRN blebs in retinal flat mounts were counted using a double-blind method, ensuring investigators were masked to the treatment groups.
Fundus Fluorescein Angiography
Mice were anesthetized with an intraperitoneal injection of 2% tribromoethanol, and pupils were dilated with 0.5% tropicamide and 0.5% phenylephrine. Each mouse received an intraperitoneal injection of 10% fluorescein sodium (Zhiyuan, Tianjin, China). The ocular fundus was observed using a fundus camera (Optoprobe Science, Glamorgan, UK; OPTO-RIS) with the OPTO-RIS Software System. Fundus fluorescein angiography (FFA) images were captured 3 minutes after the fluorescein sodium injection.
Serum and RPE Iron Content Analysis
Serum was collected as previously reported.20 Serum iron concentration was measured using the iron assay kit (Huili Biotech, Jilin, China) on the automatic biochemical analyzer (Rayto, Shenzhen, China) according to the manufacturer’s instructions. RPE/choroid complexes were homogenized in saline and centrifuged at 1000 g for 10 minutes at 4°C, and the supernatant was collected. Iron levels were determined using the iron assay kit (Servicebio, Wuhan, China) following the manufacturer's instructions.
Statistical Analysis
The Student’s t-test was used for 2-group analysis, and 1-way ANOVA was used for analysis of more than 2 groups. The analysis was performed using Prism 8 software (GraphPad, San Diego, CA, USA). Data are presented as mean ± standard error of the mean (SEM). A P value < 0.05 was considered statistically significant.
Results
Iron Metabolism is Disrupted in the Retina and RPE of Vldlr−/− Mice at p12
An appropriate animal model is needed to investigate the effects of iron in nAMD. In this study, we used the Vldlr−/− mouse model, a model of neovascular AMD.21,22 To test whether a Vldlr−/− mouse is suitable for this study, RNA-seq analysis was performed using the retina and RPE of WT and Vldlr−/− mice at p12. The heat map showed the differentially expressed genes (DEGs) in the retina and RPE of Vldlr−/− mice (Supplementary Figs. S2A, S2B), in which 34 genes were upregulated and 52 genes were downregulated in the retinas (Supplementary Fig. S2C), whereas 297 genes were upregulated and 223 genes were downregulated in RPE (Supplementary Fig. S2D). Interestingly, expression of transferrin (Trf) was downregulated in both retina and RPE among the top 10 DEGs (Figs. 1A–1D). Further, we verified the protein expression of iron metabolism-related proteins. The protein expression of transferrin (Tf) was significantly increased in the RPE of Vldlr−/− mice compared to age-matched WT mice (Figs. 1G, 1H), and an ascending trend in transferrin receptor (TfR) expression was observed in both the retina and RPE of Vldlr−/− mice (Figs. 1E–1H). Meanwhile, the expression of iron storage protein ferritin (Frt) was significantly decreased in both retina (Figs. 1E, 1F) and RPE (Figs. 1G, 1H) in Vldlr−/− mice compared to those in WT mice, indicating decreased iron storage and thus a possible increased free iron level. Taken together, these results suggest that iron homeostasis is disrupted in the retina and RPE of Vldlr−/− mice in the initial stage of retinal neovascularization, indicating that the Vldlr−/− mouse is a suitable nAMD model to study the possible involvement of iron in the pathogenesis of nAMD.
Figure 1.
Disrupted iron homeostasis in both the retina and RPE of Vldlr−/− mice at p12. (A, B) Volcano plots revealed the fold change and significance levels of DEGs in the retina (A) and RPE (B) of WT and Vldlr−/− mice at p12 with FDR < 0.05. The blue and red dots represent down- and upregulated DEGs, respectively, whereas the black dot indicates nonsignificant DEGs. Notably, Trf was found to be significantly downregulated in both retina and RPE. (C, D) The Bar chart displayed the log2 fold change in mRNA expression levels of the 10 DEGs that were significantly up- and downregulated in the retina (C, n = 3) and RPE (D, n = 4) of Vldlr−/− mice at p12 compared to those of age-matched WT mice. (E-H) The representative images of Western blot showed the protein levels of Tf, TfR, and Frt (normalized to GAPDH) in the retina (E, F) and RPE (G, H) of WT and Vldlr−/− mice (n = 6) at p12. RPE, retinal pigment epithelium; FDR, false discovery rate; Trf and Tf, transferrin; TfR, transferrin receptor; Frt, ferritin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Data are shown as mean ± SEM. *P < 0.05, ***P < 0.001. Two-tailed Student's t-test was used.
Iron Homeostasis is Altered in the Retina, RPE, and Serum in Vldlr−/− Mice at p21
Previous studies have shown that fundus fluorescein leakage spots were first observed in Vldlr−/− mice at p21 through FFA.21,23 To further investigate the association between iron metabolism and retinal neovascularization in Vldlr−/− mice, we examined the protein levels of Tf, TfR, and Frt in the RPE and retina at p21. Levels of Tf and TfR were upregulated, and levels of Frt were downregulated in the retina (Figs. 2A, 2B) and RPE (Figs. 2C, 2D) of Vldlr−/− mice. The levels of iron were also elevated in the serum of Vldlr−/− mice (Fig. 2E). In addition, immunostaining of retinal sections showed that Tf was colocalized in the retinal neovascularization areas (Fig. 2F). Overall, our data demonstrate that Vldlr−/− mice at p21 have increased iron levels in the retina, RPE, and serum, suggesting a potential association between impaired iron homeostasis and retinal neovascularization.
Figure 2.
Iron homeostasis was altered in the retina, RPE and serum of Vldlr−/− mice at p21. (A–D) Protein levels of Tf, TfR, and Frt were measured by Western blot analysis and normalized to β-actin levels in the retina (A, B) and RPE (C, D) of WT and Vldlr−/− mice (n = 4-6) at p21. (E) Serum iron levels of WT and Vldlr−/− mice at p21 (n = 4-6) were measured. (F) Representative images of immunofluorescence staining for Tf (red) in the retinal sections of WT and Vldlr−/− mice at p21. An arrow pointed out that Tf was located in the retinal neovascularization area in Vldlr−/− mice. The nuclei were stained with DAPI (blue). Scale bar = 50 µm. DAPI, 4',6-diamidino-2-phenylindole; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Two-tailed Student's t-test was used.
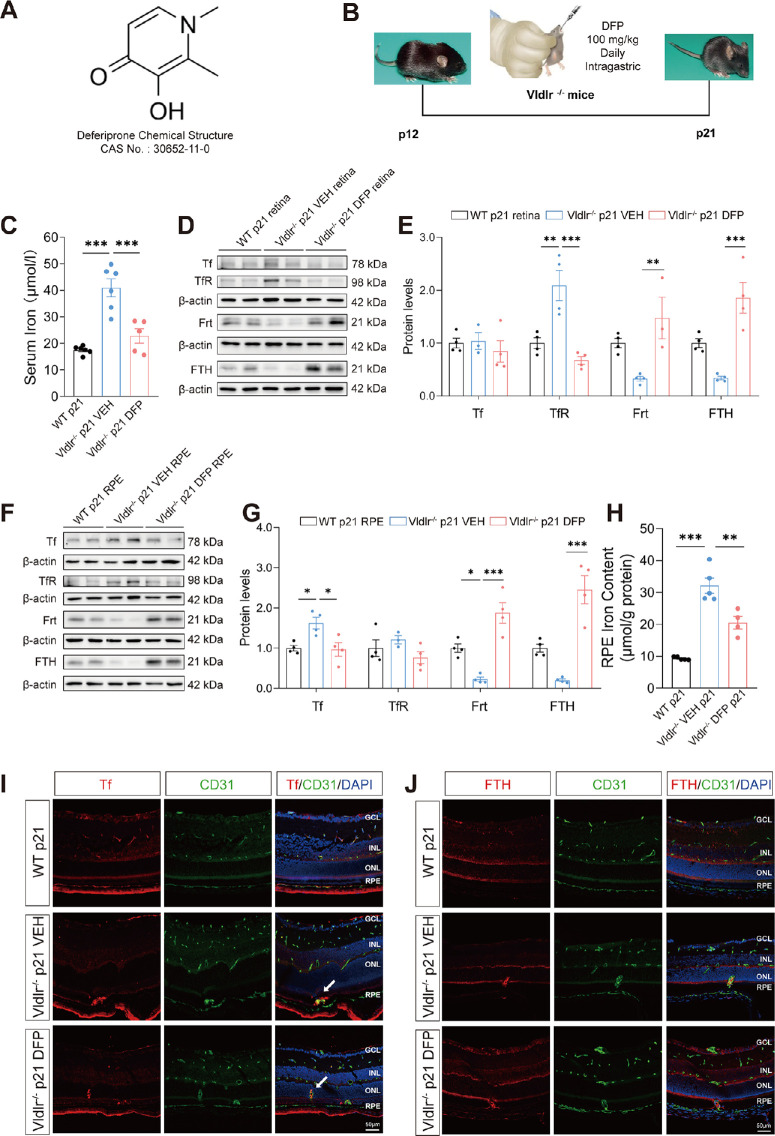
DFP Restores Iron Levels in the Retina, RPE, and Serum of Vldlr−/− Mice
To further verify whether iron metabolism contributes to the pathologic neovascularization in Vldlr−/− mice, we administered an iron chelator, DFP, to restore the iron levels (Fig. 3A). DFP is a clinically used and efficient oral iron chelator.24 DFP is capable of passing through the blood-retinal barrier (BRB) and serves as an excellent iron remover to treat retinal diseases.7 In this study, Vldlr−/− mice at p12 were fed with DFP or vehicle (saline) daily, and the retina and RPE were collected at p21 (Fig. 3B). The serum iron levels were significantly upregulated in Vldlr−/− mice and restored by DFP treatment (Fig. 3C), indicating successful delivery of DFP into Vldlr−/− mice. Levels of TfR were significantly increased in the retina of Vldlr−/− mice and restored to normal levels after DFP treatment (Figs. 3D, 3E). The Tf expression was increased in the RPE of Vldlr−/− mice and rescued to normal levels after DFP treatment (Figs. 3F, 3G). Furthermore, DFP significantly reversed the decreased Frt protein levels in the retina and RPE of Vldlr−/− mice (Figs. 3D–3G). Additionally, iron contents in the RPE were significantly reduced in Vldlr−/− mice after DFP treatment (Fig. 3H). Immunofluorescence results showed a colocalization of Tf and endothelial cells in the retinal sections of Vldlr−/− mice (Figs. 3I, 3J). The immunostaining signals of Tf, located in the retinal neovascular areas, were decreased after DFP administration in Vldlr−/− mice compared with WT mice (Fig. 3I). Vldlr−/− mice exhibited a reduced signal of ferritin heavy chains (FTH), whereas DFP treatment increased the FTH signal in the neural retina of Vldlr−/− mice (Fig. 3J), suggesting the reversed FTH retained free iron and maintained proper iron storage in the retina. Taken together, these results demonstrate that DFP treatment successfully reverses the disrupted iron homeostasis in the retina, RPE and serum of Vldlr−/− mice.
Figure 3.
Iron homeostasis was restored in Vldlr−/− mice after deferiprone (DFP) administration. (A) Chemical structure of DFP, an iron chelator. (B) Schematic diagram of mouse treatment schedule and way of delivery. Vldlr−/− mice were administered with DFP or VEH (saline) by gavage daily from p12 to p21. (C) Iron levels in the serum (n = 5-6) were measured after DFP treatment in Vldlr−/− mice. (D–G) Representative images of Western blot images of Tf, TfR, Frt, and FTH (normalized to β-actin) in the retina (D, E) and RPE (F, G) of Vldlr−/− mice treated with VEH or DFP (n = 3–4). (H) RPE iron content was measured in VEH- and DFP-treated Vldlr−/− mice. (I, J) Immunofluorescence representative images showed the location of Tf (I, red) and FTH (J, red) with CD31 (green), a marker of vascular endothelial cells. The nuclei were stained with DAPI (blue). An arrow pointed out the colocalization of Tf and CD31 in the neovascular area in panel I. Scale bar = 50 µm. DFP, deferiprone; VEH, vehicle; FTH, ferritin heavy chain; CD31, cluster of differentiation 31. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA analysis was used.
DFP Suppresses Retinal Neovascularization in Vldlr−/− Mice
Next, the effects of DFP on retinal neovascularization were investigated. Lectin staining of the whole-mounted retina revealed a reduction of intraretinal neovascular (IRN) blebs in Vldlr−/− mice after DFP administration (Figs. 4A–4C). Additionally, the results of FFA demonstrated a significant decrease in vascular leakage spots after DFP administration (Figs. 4D, 4E). The hematoxylin and eosin (H&E) staining of retinal sections showed that DFP treatment attenuated neovascularization with fewer pathologic retinal vessels in the subretinal area of Vldlr−/− mice (Fig. 4F), without significant changes of the retinal layers and structure, suggesting that DFP had no toxic effect on Vldlr−/− retinas. Taken together, these results indicate that DFP has inhibitory effects on retinal neovascularization in Vldlr−/− mice.
Figure 4.
Anti-angiogenic effects of DFP in Vldlr−/− mice. (A) Retinas from VEH- and DFP-treated Vldlr−/− mice were flat-mounted and stained with isolectin GS-IB4 (red). Scale bar = 500 µm. (B) Quantification of IRN blebs in retinal flat mounts of Vldlr−/− mice treated with VEH or DFP (n = 3) at p21. (C) Higher magnification showed IRN blebs in Vldlr−/− mice treated with VEH or DFP. Scale bar = 50 µm. (D) Representative images of fundus fluorescein angiography in Vldlr−/− mice treated with VEH and DFP. (E) Statistical plot of fundus vascular leakage spots in Vldlr−/− mice treated with VEH (n = 7) and DFP (n = 9). (F) Representative retinal section images of H&E-staining in VEH- and DFP-treated Vldlr−/− mice. Scale bar = 50 µm. IRN, intraretinal neovascular; H&E, hematoxylin and eosin. Data are shown as mean ± SEM. **P < 0.01. One-way ANOVA analysis was used.
DFP Attenuates Retinal Inflammation in Vldlr−/− Mice
The anti-inflammatory effects of DFP in the Vldlr−/− mice were further investigated. Protein levels of inflammation factors GFAP and VEGF were significantly reduced in DFP-treated Vldlr−/− retinas compared to VEH-treated Vldlr−/− retinas (Figs. 5A, 5B). In addition, mRNA levels of pro-inflammatory factors interleukin-1β (IL-1β), vascular cell adhesion molecule-1 (VCAM-1), and tumor necrosis factor-α (TNF-α) were downregulated in DFP-treated Vldlr−/− retinas (Figs. 5C–5E). Taken together, these results indicate that DFP suppresses retinal inflammation in Vldlr−/− mice.
Figure 5.
Anti-inflammatory effects of DFP in the retina of Vldlr−/− mice. (A, B) The representative images of Western blot analysis of GFAP and VEGF (normalized to β-actin) in the retina of Vldlr−/− mice treated with VEH or DFP (n = 4). (C–E) Real-time PCR of IL-1β (C), VCAM-1 (D), and TNF-α (E) mRNA expression (normalized to β-actin) in VEH- and DFP-treated Vldlr−/− retinas (n = 4). GFAP, glial fibrillary acidic protein; VEGF, vascular endothelial growth factor; IL-1β, interleukin-1β; VCAM-1, vascular cell adhesion molecule-1; TNF-α, tumor necrosis factor-α. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA analysis and two-tailed Student's t-test were used.
DFP Suppresses Macrophage Recruitment in the RPE of Vldlr−/− Mice
Macrophages are the primary inflammatory cells in the retina. Studies have shown that macrophages are recruited to the subretinal space during the development of AMD.25 To investigate the involvement of macrophage in iron-related retinal neovascularization, we performed F4/80 staining on retinal sections and RPE/choroid complex flat mounts. Immunostaining of the retinal sections showed that F4/80+ macrophages were increased in the subretinal space between the outer nuclear layer (ONL) and RPE in VEH-treated Vldlr−/− mice (Figs. 6A, 6B), which is colocalized with Tf expression; and were decreased at the subretinal retinal space and lesion site in DFP-treated Vldlr−/− mice (Figs. 6A, 6B). Moreover, DFP-treated Vldlr−/− mice displayed lower coverage of F4/80+ macrophages in lectin+ neovascular areas in the RPE/choroid complex compared to that of VEH-treated Vldlr−/− mice (Figs. 6C, 6D). F4/80 is a protein encoded by the adhesion G protein-coupled receptor E1 (ADGRE1) gene.26 The mRNA level of ADGRE1 was downregulated in DFP-treated Vldlr−/− retinas (Fig. 6E). Taken together, the results indicate that DFP suppresses macrophage recruitment at neovascular lesions in the retina and RPE of Vldlr−/− mice.
Figure 6.
Decreased F4/80+ macrophage infiltration in the retinal neovascular area of Vldlr−/− mice treated with DFP. (A) Representative images of immunofluorescence staining for F4/80 (red), Tf (green), and DAPI (blue) in the retina sections of Vldlr−/− mice treated with VEH or DFP. Scale bar = 50 µm. (B) Pixel intensity of F4/80 immunohistochemistry was quantified in retinal sections of the VEH- and DFP-treated Vldlr−/− mice (n = 3–4) at p21. (C) Representative images of isolectin (red) and F4/80 (green) immunostaining in RPE/choroid complex flat mounts from Vldlr−/− mice treated with VEH and DFP. Cell nuclei were stained with DAPI (blue). Scale bar = 50 µm. (D) Percentage of pixels of F4/80+ isolectin+ area to total isolectin+ area in subretinal neovascular lesions of VEH- and DFP-treated Vldlr−/− mice (n = 6) at p21. (E) Real-time PCR of ADGRE1 mRNA expression (normalized to β-actin) in VEH- and DFP-treated Vldlr−/− retinas (n = 4). ADGRE1, adhesion G protein-coupled receptor E1. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA analysis and two-tailed Student's t-test were used.
Ferroptosis is Not Involved in the Pathogenesis of Retinal Neovascularization in Vldlr−/− Mice
Excessive iron accumulation causes oxidative stress and lipid peroxidation, leading to ferroptosis, a form of iron-dependent cell death.27 Recently, ferroptosis has been reported to play important roles in the pathogenesis of neovascularization in laser-induced choroidal neovascularization (CNV)28 and oxygen-induced retinopathy.29 Thus, we investigated whether ferroptosis contributed to retinal neovascularization in Vldlr−/− mice. Expression of ferroptosis-related proteins Acyl-CoA synthetase long-chain family member 4 (ACSL4), glutathione peroxidase 4 (GPX4), and solute carrier family 7a member 11 (SLC7A11) remained unchanged in the retina of Vldlr−/− mice compared to the control mice (Supplementary Figs. S3A, S3B). Furthermore, DFP treatment had no significant changes in ferroptosis-related proteins in Vldlr−/− mice (Supplementary Figs. S3A, S3B). Therefore, iron disruption and accumulation did not induce ferroptosis in Vldlr−/− retinas.
DFP Inhibits the IL-6/JAK2/STAT3 Signaling Pathway
To further explore the possible mechanism underlying retinal inflammation and neovascularization in Vldlr−/− mice, RNA-seq analysis was performed on the retina and RPE of WT and Vldlr−/− mice at p21, respectively. The bar chart (Figs. 7A, 7B) showed the differential genes in the retina and RPE in Vldlr−/− mice compared to WT mice. In the RPE, 267 genes were upregulated, and 468 genes were downregulated (Fig. 7A); in the retina, 486 genes were upregulated, and 454 genes were downregulated (Fig. 7B). KEGG enrichment results revealed the top 20 enriched pathways in the RPE of WT and Vldlr−/− mice (Fig. 7C). The DEGs were enriched in several common KEGG pathways in the RPE with high light of the JAK-STAT3 pathway (Fig. 7C). Subsequently, a PPI network of DEGs based on the top nine KEGG pathways in the retina between WT and Vldlr−/− mice was constructed (Fig. 7D). Interestingly, iron metabolism-related proteins, such as transferrin receptor (Tfrc) and caveolin-1 (Cav1),11,30 were closely associated with the JAK-STAT pathway (Fig. 7D). A significant upregulation of IL-6 and phosphorylated-JAK2 (p-JAK2) levels was detected in the retina and RPE, respectively, in Vldlr−/− mice (Figs. 7E–7H). Protein expression of phosphorylated-STAT3 (p-STAT3) was dramatically elevated in the retina (Figs. 7E, 7F) and RPE (Figs. 7G, 7H) of Vldlr−/− mice. DFP treatment significantly downregulated the expression of IL-6, p-JAK2, and p-STAT3 (Figs. 7E–7H) in the retina and RPE of Vldlr−/− mice. These findings demonstrated that IL-6-JAK2-STAT3 signaling is activated in the Vldlr−/− retinas, and DFP can reverse the upregulated IL-6/JAK2/STAT3 signaling, suggesting that the IL-6/JAK2/STAT3 pathway may be involved in iron overload related retinal inflammation and neovascularization.
Figure 7.
The suppression of IL6/JAK2/STAT3 signaling in the retina and RPE of Vldlr−/− mice after DFP treatment. (A, B) RNA-Seq analysis revealed the DEGs in both the RPE (A) and the retina (B) of WT and Vldlr−/− mice at p21 (n = 3). (C) KEGG enrichment pathways of DEGs in RPE of Vldlr−/− mice compared with WT showed that 20 pathways were significantly enriched. Low q-values are in red, and high q-values are in blue; the size of the circle is proportional to the number of enriched genes. (D) Protein-protein interaction (PPI) networks of DEGs involved in the top 9 KEGG enrichment pathways in the retina of WT and Vldlr−/− mice at p21. Inflammatory and iron-related factors are marked. (E–H) Representative images and quantifications of Western blot analysis of protein levels of IL-6, p-STAT3, and STAT3 (normalized to β-actin) in the retina (E, F), and p-JAK2, JAK2, p-STAT3, STAT3 (normalized to β-actin) in the RPE (G, H) of Vldlr−/− mice treated with VEH or DFP (n = 4). KEGG, Kyoto encyclopedia of genes and genomes; VEGFA, vascular endothelial growth factor A; Tfrc, transferrin receptor; JAK3, Janus kinase 3; STAT3, signal transducer and activator of transcription 3; Cav1, caveolin-1; IL-6, interleukin-6; p-STAT3, phosphorylated-STAT3; JAK2, Janus kinase 2; p-JAK2, phosphorylated-JAK2. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01. One-way ANOVA analysis was used.
Discussion
Iron homeostasis is essential for normal cell function. Multiple studies have indicated that abnormal iron levels are associated with AMD. In this study, we have found that Vldlr−/− mice, a murine model of nAMD, have systemic iron overload and increased Tf in the retina and RPE, which mimic the abnormal iron levels in patients with AMD.10,31,32 The application of iron chelator DFP suppresses retinal neovascularization and vascular leakage, indicating the important role of iron in retinal neovascularization. Moreover, the inhibitory effects of DFP on retinal neovascularization are possibly through reducing the inflammatory cytokines and inhibiting the recruitment of macrophages. Further, the IL-6/JAK2/STAT3 signaling pathway is inhibited by DFP in Vldlr−/− mice, indicating the possible mechanism of the action of DFP. Overall, the present study demonstrates that disrupted iron metabolism may contribute to retinal neovascularization in nAMD, and restoring iron homeostasis by DFP could be a potential therapeutic strategy for nAMD.
The Vldlr−/− mouse is an animal model for retinal angiomatous proliferation (RAP), which accounts for approximately 15% of patients with nAMD.33 RAP originates from the retinal vessels and grows toward the subretinal space.21 Pathological blood vessels originate in the OPL and extend through the ONL as early as p12 in Vldlr−/− mice.22,23 Progressive IRN blebs and the vascular leakage spots of FFA were first observed at p21.21,23
Intracellular iron levels are regulated through three main mechanisms: modulation of TfR expression to control iron uptake, adjustment of Frt expression to manage the labile iron pool, and alteration of ferroportin (Fpn) expression to regulate iron export. Tf, the only protein that binds to TfR, regulates iron uptake in conjunction with TfR, whereas Frt, the major iron storage protein, influences cellular iron levels.34 In addition, significant alterations in Tf, TfR, Frt, and Fpn levels are found in several retinal diseases associated with iron accumulation.31,32,35–37 Consequently, our study focused on Tf, TfR, and Frt to investigate iron metabolism in the retina and RPE. The protein levels of iron-related proteins Tf, TfR, and Frt were significantly altered at p12, with more pronounced changes at p21, indicating a persistent and progressive disruption of iron homeostasis in Vldlr−/− mice. Thus, Vldlr−/− mice could be an appropriate model to investigate the involvement of iron in the pathogenesis of nAMD. Meanwhile, to our knowledge, elevated iron levels in the retina and RPE in an nAMD animal model are first reported.
In this study, bulk-RNA sequencing showed Trf mRNA expression was downregulated, whereas the verified protein levels of Tf were upregulated in both the retina and RPE of Vldlr−/− mice. The discrepancy in Tf between mRNA and protein levels may suggest that cells may try to dampen the elevated Tf protein expression by decreasing mRNA levels of Tf, thus negatively regulating Trf gene expression. Further study is needed to verify this negative regulation.
The inflammatory response is considered a major cause of pathological angiogenesis.6,38 Macrophages, closely associated with iron metabolism, are the primary inflammatory cells.39 Macrophages are recruited by inflammatory chemokines and have the ability to release antigenic cytokines and promote retinal angiogenesis.40,41 Additionally, macrophages degrade the ECM to create space for the proliferation of neovascular endothelial cells.42,43 Studies have shown that excessive iron alters macrophages toward an activated and inflammatory phenotype, leading to the secretion of inflammatory cytokines, such as IL-6, TNF-α, etc.39,44 In this study, we observed an increased number of macrophages and elevated Tf expression with colocalization of macrophages and Tf at the neovascular area in the Vldlr−/− retinal sections. Moreover, macrophages were recruited at the neovascular area, as shown in the flat-mounted RPE/choroid complex, suggesting that iron overload may recruit macrophages to the vascular lesion sites, resulting in the release of inflammatory mediators and promotion of retinal angiogenesis in the Vldlr−/− mice.
DFP has been reported to attenuate retinal degeneration in several animal models, such as NaIO3-treated mice, hepcidin knockout mice, Cp and Heph double knockout mice, and the rd10 model of retinitis pigmentosa.45–48 However, the effects of DFP on nAMD were still unknown. Our results demonstrated that DFP restored iron homeostasis in Vldlr−/− mice and had no toxic effects on the retina, as indicated by H&E staining. DFP administration reduced IRN blebs and retinal vascular leakage spots in Vldlr−/− mice. These results suggest that DFP suppresses retinal neovascularization, and the overloaded iron may contribute to retinal neovascularization in Vldlr−/− mice. In this study, DFP administration decreased the protein levels of Tf and TfR, but increased the protein levels of Frt in the retina and RPE of Vldlr−/− mice. In contrast, other studies reported that iron chelators decreased Frt and increased Tf.47,49,50 This discrepancy may be due to the activated and recruited macrophages in the lesion sites. It is possible that DFP might suppress the activation and iron uptake in macrophages and microglia, thereby affecting the availability of iron to other retinal cells.
It has been reported that iron overload can stimulate the JAK2/STAT3 signaling pathway in triple-negative breast cancer cells.51 Hepcidin, an important regulator of iron metabolism, is regulated by the JAK2/STAT3 pathway.52 In this study, we have found that IL-6/JAK2/STAT3 signaling was activated in the retina of Vldlr−/− mice and suppressed by DFP. These results are supported by several studies.53–57 For example, a recent study has shown that DFP blocked the production of IL-6, an upstream of STAT3, in pro-inflammatory conditions.53 In addition, iron chelator deferoxamine was reported to suppress STAT3 activity and alleviate macrophage infiltration by inhibiting inflammation.54–57 Therefore, it is possible that the anti-inflammatory effects of DFP in the retina are partly through inhibiting STAT3 activation and suppressing macrophage recruitment. Further studies are warranted to study the potential association of STAT3 and macrophages in retinal neovascularization.
In summary, we have demonstrated disturbed iron homeostasis in the retina, RPE, and serum of Vldlr−/− mice. DFP can suppress retinal neovascularization by inhibiting retinal inflammation, reducing macrophage recruitment, and suppressing IL-6/JAK2/STAT3 signaling. Our study suggests that iron may be involved in the development or pathogenesis of retinal neovascularization, and maintaining iron homeostasis may serve as a potential treatment for nAMD.
Supplementary Material
Acknowledgments
Supported by the Fundamental Research Funds for Xiamen University (grant NO. 20720220056), the Natural Science Youth Foundation of Xiamen City (grant NO. 3502Z202372007), and the Natural Science Foundation of Fujian Province (grant NO. 2023J011585).
Disclosure: Y. Xu, None; S. Huang, None; S. Zhou, None; X. Wang, None; M. Wei, None; X. Chen, None; R. Zong, None; X. Lin, None; S. Li, None; Z. Liu, None; Q. Chen, None
References
- 1. Guymer RH, Campbell TG.. Age-related macular degeneration. Lancet (London, England). 2023; 401: 1459–1472. [DOI] [PubMed] [Google Scholar]
- 2. Wolf AT, Harris A, Oddone F, Siesky B, Verticchio Vercellin A, Ciulla TA. Disease progression pathways of wet AMD: opportunities for new target discovery. Expert Opin Ther Targets. 2022; 26: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mu Q, Chen L, Gao X, et al.. The role of iron homeostasis in remodeling immune function and regulating inflammatory disease. Sci Bull. 2021; 66: 1806–1816. [DOI] [PubMed] [Google Scholar]
- 4. Muhoberac BB, Vidal R. Iron, ferritin, hereditary ferritinopathy, and neurodegeneration. Front Neurosci. 2019; 13: 1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urrutia PJ, Mena NP, Núñez MT.. The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front Pharmacol. 2014; 5: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Szczepan M, Llorián-Salvador M, Chen M, Xu H.. Immune cells in subretinal wound healing and fibrosis. Front Cell Neurosci. 2022; 16: 916719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao F, Peng J, Zhang E, et al.. Pathologically high intraocular pressure disturbs normal iron homeostasis and leads to retinal ganglion cell ferroptosis in glaucoma. Cell Death Differ. 2023; 30: 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daruich A, Le Rouzic Q, Jonet L, et al.. Iron is neurotoxic in retinal detachment and transferrin confers neuroprotection. Sci Adv. 2019; 5: eaau9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jünemann AG, Stopa P, Michalke B, et al.. Levels of aqueous humor trace elements in patients with non-exsudative age-related macular degeneration: a case-control study. PLoS One. 2013; 8: e56734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biesemeier A, Yoeruek E, Eibl O, Schraermeyer U.. Iron accumulation in Bruch's membrane and melanosomes of donor eyes with age-related macular degeneration. Exp Eye Res. 2015; 137: 39–49. [DOI] [PubMed] [Google Scholar]
- 11. Deng GH, Wu CF, Li YJ, et al.. Caveolin-1 is critical for hepatic iron storage capacity in the development of nonalcoholic fatty liver disease. Military Med Res. 2023; 10: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X, Li M, Diao K, et al.. Deferoxamine attenuates visual impairment in retinal ischemia‒reperfusion via inhibiting ferroptosis. Sci Rep. 2023; 13: 20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Picard E, Jonet L, Sergeant C, et al.. Overexpressed or intraperitoneally injected human transferrin prevents photoreceptor degeneration in rd10 mice. Mol Vis. 2010; 16: 2612–2625. [PMC free article] [PubMed] [Google Scholar]
- 14. Picard E, Le Rouzic Q, Oudar A, et al.. Targeting iron-mediated retinal degeneration by local delivery of transferrin. Free Radical Biol Med. 2015; 89: 1105–1121. [DOI] [PubMed] [Google Scholar]
- 15. Murray AR, Chen Q, Takahashi Y, Zhou KK, Park K, Ma JX.. MicroRNA-200b downregulates oxidation resistance 1 (Oxr1) expression in the retina of type 1 diabetes model. Invest Ophthalmol Vis Sci. 2013; 54: 1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanehisa M, Araki M, Goto S, et al.. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008; 36: D480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He X, Wang S, Sun H, et al.. Lacrimal gland microenvironment changes after obstruction of lacrimal gland ducts. Invest Ophthalmol Vis Sci. 2022; 63: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mao Y, Ou S, Zhu C, et al.. Downregulation of p38 MAPK signaling pathway ameliorates tissue-engineered corneal epithelium. Tissue Eng Part A. 2022; 28: 977–989. [DOI] [PubMed] [Google Scholar]
- 19. Connor KM, Krah NM, Dennison RJ, et al.. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009; 4: 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li X, He F, Tuo X, et al.. Electroacupuncture ameliorates peptic ulcer disease in association with gastroduodenal microbiota modulation in mice. Front Cell Infect Microbiol. 2022; 12: 935681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu W, Jiang A, Liang J, et al.. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest Ophthalmol Vis Sci. 2008; 49: 407–415. [DOI] [PubMed] [Google Scholar]
- 22. Joyal JS, Sun Y, Gantner ML, et al.. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat Med. 2016; 22: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson V, Xiang M, Chen Z, Junge HJ.. Neurite mistargeting and inverse order of intraretinal vascular plexus formation precede subretinal vascularization in Vldlr mutant mice. PLoS One. 2015; 10: e0132013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Entezari S, Haghi SM, Norouzkhani N, et al.. Iron chelators in treatment of iron overload. J Toxicol. 2022; 2022: 4911205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaur G, Singh NK.. Inflammation and retinal degenerative diseases. Neural Regen Res. 2023; 18: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamann J, Aust G, Araç D, et al.. International union of basic and clinical pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol Rev. 2015; 67: 338–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang WS, Stockwell BR.. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016; 26: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao X, Gao M, Liang J, et al.. SLC7A11 reduces laser-induced choroidal neovascularization by inhibiting RPE ferroptosis and VEGF production. Front Cell Devel Biol. 2021; 9: 639851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu CQ, Liu XY, Ouyang PW, et al.. Ferrostatin-1 attenuates pathological angiogenesis in oxygen-induced retinopathy via inhibition of ferroptosis. Exp Eye Res. 2023; 226: 109347. [DOI] [PubMed] [Google Scholar]
- 30. Tang W, Li Y, He S, et al.. Caveolin-1 alleviates diabetes-associated cognitive dysfunction through modulating neuronal ferroptosis-mediated mitochondrial homeostasis. Antioxid Redox Signal. 2022; 37: 867–886. [DOI] [PubMed] [Google Scholar]
- 31. Chowers I, Wong R, Dentchev T, et al.. The iron carrier transferrin is upregulated in retinas from patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006; 47: 2135–2140. [DOI] [PubMed] [Google Scholar]
- 32. Hahn P, Milam AH, Dunaief JL.. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch's membrane. Arch Ophthalmol (Chicago, Ill: 1960). 2003; 121: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 33. Heckel E, Cagnone G, Agnihotri T, et al.. Triglyceride-derived fatty acids reduce autophagy in a model of retinal angiomatous proliferation. JCI Insight. 2022; 7: e154174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao T, Guo X, Sun Y.. Iron accumulation and lipid peroxidation in the aging retina: implication of ferroptosis in age-related macular degeneration. Aging Dis. 2021; 12: 529–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dentchev T, Hahn P, Dunaief JL.. Strong labeling for iron and the iron-handling proteins ferritin and ferroportin in the photoreceptor layer in age-related macular degeneration. Arch Ophthalmol (Chicago, Ill: 1960). 2005; 123: 1745–1746. [DOI] [PubMed] [Google Scholar]
- 36. Sharon D, Blackshaw S, Cepko CL, Dryja TP.. Profile of the genes expressed in the human peripheral retina, macula, and retinal pigment epithelium determined through serial analysis of gene expression (SAGE). Proc Natl Acad Sci USA. 2002; 99: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen H, Liu B, Lukas TJ, Suyeoka G, Wu G, Neufeld AH.. Changes in iron-regulatory proteins in the aged rodent neural retina. Neurobiol Aging. 2009; 30: 1865–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang L, Xu GT, Zhang JF.. Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural Regen Res. 2023; 18: 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ni S, Yuan Y, Kuang Y, Li X.. Iron metabolism and immune regulation. Front Immunol. 2022; 13: 816282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davies MH, Eubanks JP, Powers MR.. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol Vis. 2006; 12: 467–477. [PubMed] [Google Scholar]
- 41. Gao X, Wang YS, Li XQ, et al.. Macrophages promote vasculogenesis of retinal neovascularization in an oxygen-induced retinopathy model in mice. Cell Tissue Res. 2016; 364: 599–610. [DOI] [PubMed] [Google Scholar]
- 42. Duffield JS, Lupher M, Thannickal VJ, Wynn TA.. Host responses in tissue repair and fibrosis. Ann Rev Pathol. 2013; 8: 241–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laskin DL, Sunil VR, Gardner CR, Laskin JD.. Macrophages and tissue injury: agents of defense or destruction? Ann Rev Pharmacol Toxicol. 2011; 51: 267–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DeRosa A, Leftin A.. The iron curtain: macrophages at the interface of systemic and microenvironmental iron metabolism and immune response in cancer. Front Immunol. 2021; 12: 614294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hadziahmetovic M, Pajic M, Grieco S, et al.. The oral iron chelator deferiprone protects against retinal degeneration induced through diverse mechanisms. Transl Vis Sci Technol. 2012; 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hadziahmetovic M, Song Y, Wolkow N, et al.. The oral iron chelator deferiprone protects against iron overload-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song D, Zhao L, Li Y, et al.. The oral iron chelator deferiprone protects against systemic iron overload-induced retinal degeneration in hepcidin knockout mice. Invest Ophthalmol Vis Sci. 2014; 55: 4525–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Obolensky A, Berenshtein E, Lederman M, et al.. Zinc-desferrioxamine attenuates retinal degeneration in the rd10 mouse model of retinitis pigmentosa. Free Radic Biol Med. 2011; 51: 1482–1491. [DOI] [PubMed] [Google Scholar]
- 49. Wang K, Peng B, Xiao J, Weinreb O, Youdim MBH, Lin B.. Iron-chelating drugs enhance cone photoreceptor survival in a mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2017; 58: 5287–5297. [DOI] [PubMed] [Google Scholar]
- 50. Zou C, Liu X, Xie R, et al.. Deferiprone attenuates inflammation and myocardial fibrosis in diabetic cardiomyopathy rats. Biochem Biophys Res Commun. 2017; 486: 930–936. [DOI] [PubMed] [Google Scholar]
- 51. Cheng M, Liu P, Xu LX.. Iron promotes breast cancer cell migration via IL-6/JAK2/STAT3 signaling pathways in a paracrine or autocrine IL-6-rich inflammatory environment. J Inorg Biochem. 2020; 210: 111159. [DOI] [PubMed] [Google Scholar]
- 52. Rah B, Farhat NM, Hamad M, Muhammad JS.. JAK/STAT signaling and cellular iron metabolism in hepatocellular carcinoma: therapeutic implications. Clin Exp Med. 2023; 23: 3147–3157. [DOI] [PubMed] [Google Scholar]
- 53. Ramezanpour M, Smith JLP, Ooi ML, et al.. Deferiprone has anti-inflammatory properties and reduces fibroblast migration in vitro. Sci Rep. 2019; 9: 2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lui GY, Kovacevic Z, VM S, et al.. Novel thiosemicarbazones regulate the signal transducer and activator of transcription 3 (STAT3) pathway: inhibition of constitutive and interleukin 6-induced activation by iron depletion. Mol Pharmacol. 2015; 87: 543–560. [DOI] [PubMed] [Google Scholar]
- 55. Ikeda Y, Ozono I, Tajima S, et al.. Iron chelation by deferoxamine prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. PLoS One. 2014; 9: e89355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang WJ, Wei H, Frei B.. The iron chelator, desferrioxamine, reduces inflammation and atherosclerotic lesion development in experimental mice. Exp Biol Med (Maywood, NJ). 2010; 235: 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jia H, Liu X, Cao Y, et al.. Deferoxamine ameliorates neurological dysfunction by inhibiting ferroptosis and neuroinflammation after traumatic brain injury. Brain Res. 2023; 1812: 148383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.