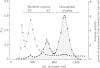
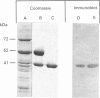
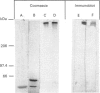
Abstract
A 52 kDa protein could only be co-purified with the CoA-modified forms of acetyl-CoA acetyltransferase (acetoacetyl-CoA thiolase) (EC 2.3.1.9) from rat liver mitochondria. Immunoprecipitations of these modified forms with anti-(acetyl-CoA acetyltransferase) IgG or anti-(52 kDa protein) IgG yielded, in addition to the appropriate proteins, the 52 kDa protein or the CoA-modified form of acetyl-CoA acetyltransferase (41 kDa) respectively. This was demonstrated by SDS/PAGE and immunoblots. The modified forms containing the 52 kDa protein could be cross-linked by 1,5-difluoro-2,4-dinitrobenzene to a high-molecular-mass complex containing both the 41 kDa and 52 kDa proteins. The 52 kDa protein was identified as mitochondrial glutamate dehydrogenase (EC 1.4.1.3) by amino acid sequence analysis. The results of co-immunoprecipitation and cross-linking characterize the CoA-modified forms of acetyl-CoA acetyltransferase and the glutamate dehydrogenase as nearest-neighbour proteins.
Full text
PDF
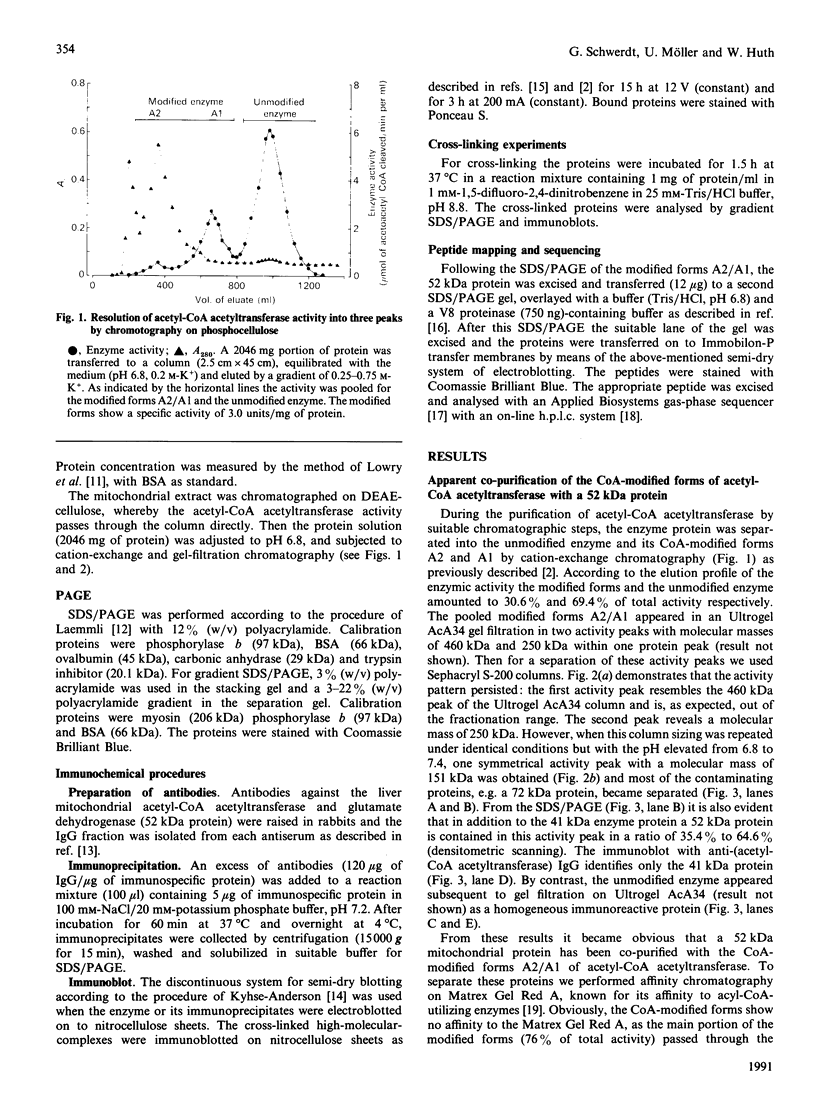


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amuro N., Ooki K., Ito A., Goto Y., Okazaki T. Nucleotide sequence of rat liver glutamate dehydrogenase cDNA. Nucleic Acids Res. 1989 Mar 25;17(6):2356–2356. doi: 10.1093/nar/17.6.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Fahien L. A., Kmiotek E. H., Marshall M. Interactions between carbamyl phosphate synthase-I-mitochondrial aspartate aminotransferase and palmitoyl-CoA. Arch Biochem Biophys. 1984 Apr;230(1):213–221. doi: 10.1016/0003-9861(84)90102-4. [DOI] [PubMed] [Google Scholar]
- Fahien L. A., Kmiotek E. H., Woldegiorgis G., Evenson M., Shrago E., Marshall M. Regulation of aminotransferase-glutamate dehydrogenase interactions by carbamyl phosphate synthase-I, Mg2+ plus leucine versus citrate and malate. J Biol Chem. 1985 May 25;260(10):6069–6079. [PubMed] [Google Scholar]
- Fahien L. A., MacDonald M. J., Teller J. K., Fibich B., Fahien C. M. Kinetic advantages of hetero-enzyme complexes with glutamate dehydrogenase and the alpha-ketoglutarate dehydrogenase complex. J Biol Chem. 1989 Jul 25;264(21):12303–12312. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Huth W., Alves F. Mitochondrial acetyl-CoA acetyltransferase in various organs from rat: form patterns and coenzyme-A-mediated modification. Biochim Biophys Acta. 1985 Aug 23;830(3):274–281. doi: 10.1016/0167-4838(85)90283-3. [DOI] [PubMed] [Google Scholar]
- Huth W., Arvand M., Möller U. Identification of [1-14C]pantothenic-acid-mediated modified mitochondrial proteins. Eur J Biochem. 1988 Mar 15;172(3):607–614. doi: 10.1111/j.1432-1033.1988.tb13932.x. [DOI] [PubMed] [Google Scholar]
- Huth W., Jonas R., Wunderlich I., Seubert W. On the mechanism of ketogenesis and its control. Purification, kinetic mechanism and regulation of different forms of mitochondrial acetoacetyl-CoA thiolases from ox liver. Eur J Biochem. 1975 Nov 15;59(2):475–489. doi: 10.1111/j.1432-1033.1975.tb02476.x. [DOI] [PubMed] [Google Scholar]
- Huth W., Worm-Breitgoff C., Möller U., Wunderlich I. Evidence for an in vivo modification of mitochondrial proteins by coenzyme A. Biochim Biophys Acta. 1991 Mar 8;1077(1):1–10. doi: 10.1016/0167-4838(91)90518-5. [DOI] [PubMed] [Google Scholar]
- Jonas R., Huth W. Acetyl-CoA acetyltransferase from bovine liver mitochondria. Molecular properties of multiple forms. Biochim Biophys Acta. 1978 Dec 8;527(2):379–390. doi: 10.1016/0005-2744(78)90352-2. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas P. L., Brody S. Neurospora crassa mitochondria contain two forms of a 4'-phosphopantetheine-modified protein. J Biol Chem. 1986 Apr 15;261(11):4785–4788. [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Ozasa H., Furuta S., Miyazawa S., Osumi T., Hashimoto T., Mori M., Miura S., Tatibana M. Biosynthesis of enzymes of rat-liver mitochondrial beta-oxidation. Eur J Biochem. 1984 Nov 2;144(3):453–458. doi: 10.1111/j.1432-1033.1984.tb08487.x. [DOI] [PubMed] [Google Scholar]
- Quandt L., Huth W. Modulation of rat-liver mitochondrial acetyl-CoA acetyltransferase activity by a reversible chemical modification with coenzyme A. Biochim Biophys Acta. 1984 Jan 31;784(2-3):168–176. doi: 10.1016/0167-4838(84)90124-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez H., Kohr W. J., Harkins R. N. Design and operation of a completely automated Beckman microsequencer. Anal Biochem. 1984 Aug 1;140(2):538–547. doi: 10.1016/0003-2697(84)90205-7. [DOI] [PubMed] [Google Scholar]
- Schwabe D., Huth W. Immunochemical aspects, molecular and kinetic properties of multiple forms of acetyl-CoA acetyltransferase from rat liver mitochondria. Biochim Biophys Acta. 1979 Oct 26;575(1):112–120. doi: 10.1016/0005-2760(79)90136-x. [DOI] [PubMed] [Google Scholar]
- Swaney J. B. Use of cross-linking reagents to study lipoprotein structure. Methods Enzymol. 1986;128:613–626. doi: 10.1016/0076-6879(86)28095-7. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D., Ridley J. Affinity chromatography of acyl-CoA utilizing enzymes on Procion Red-agarose. Biochem Biophys Res Commun. 1983 May 16;112(3):1021–1026. doi: 10.1016/0006-291x(83)91720-5. [DOI] [PubMed] [Google Scholar]
- West S. M., Price N. C. The unfolding and refolding of glutamate dehydrogenases from bovine liver, baker's yeast and Clostridium symbosium. Biochem J. 1988 Apr 1;251(1):135–139. doi: 10.1042/bj2510135. [DOI] [PMC free article] [PubMed] [Google Scholar]