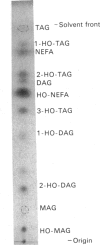
Abstract
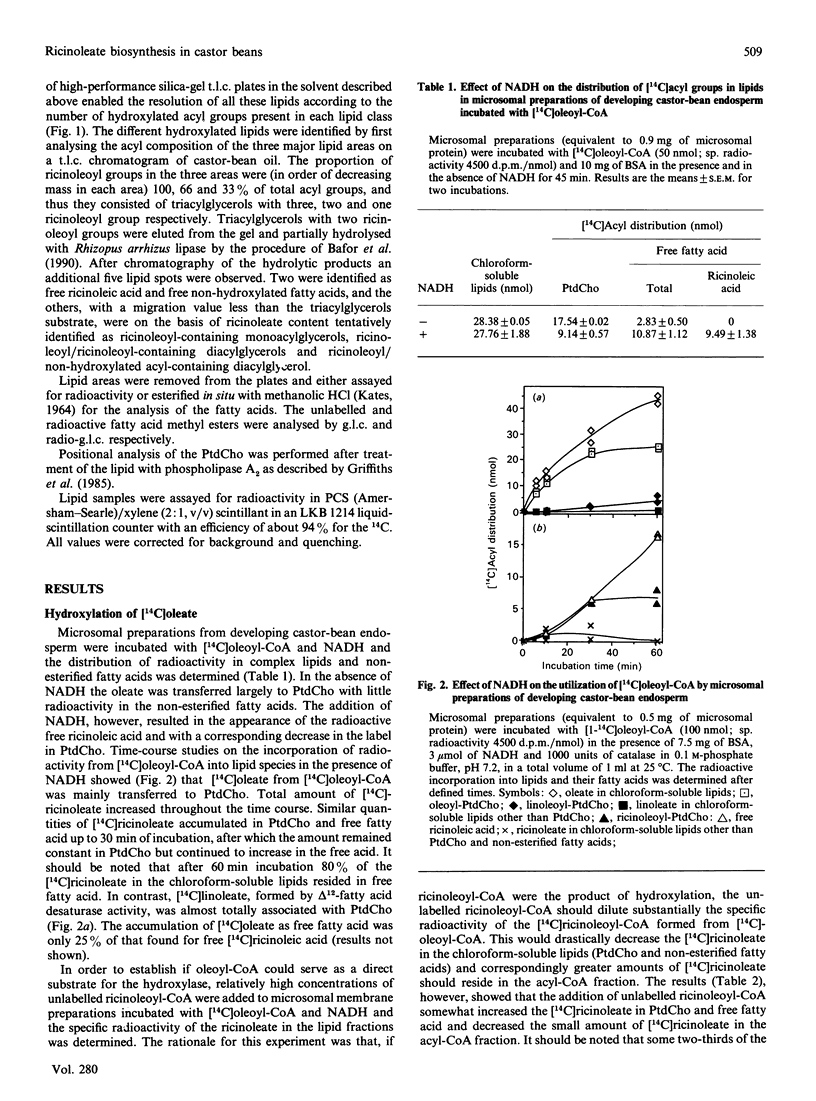
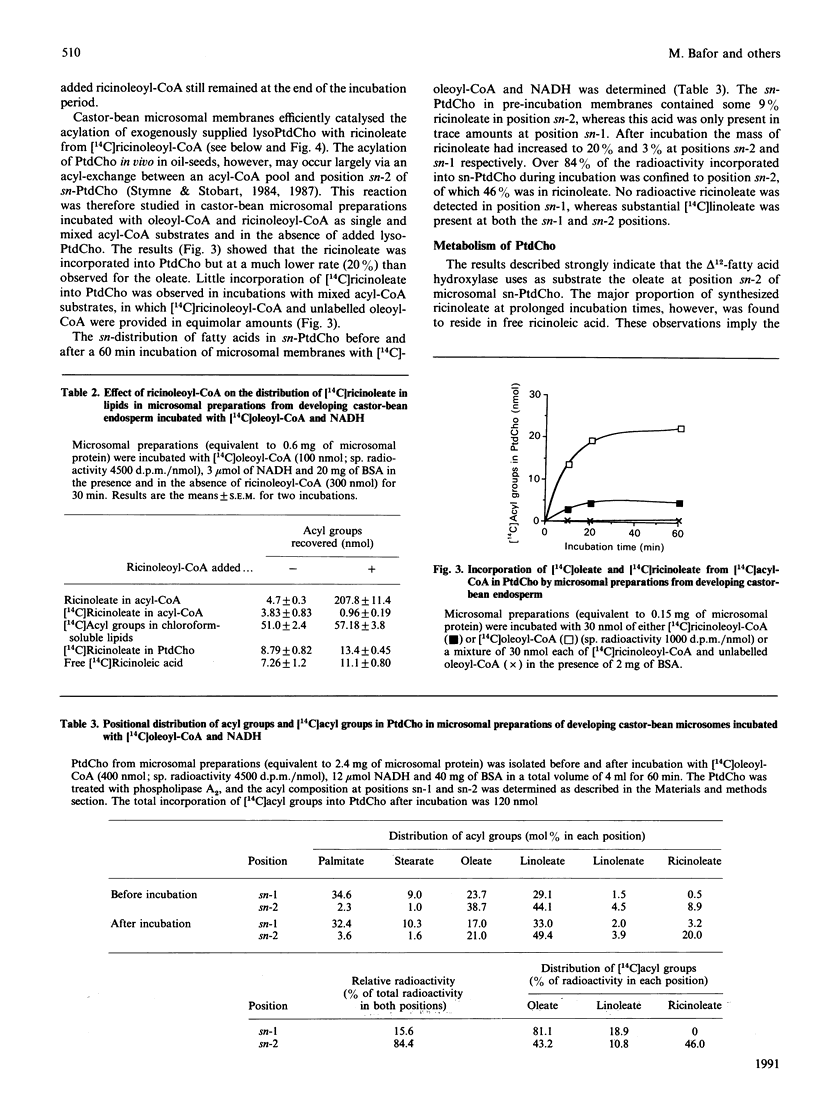
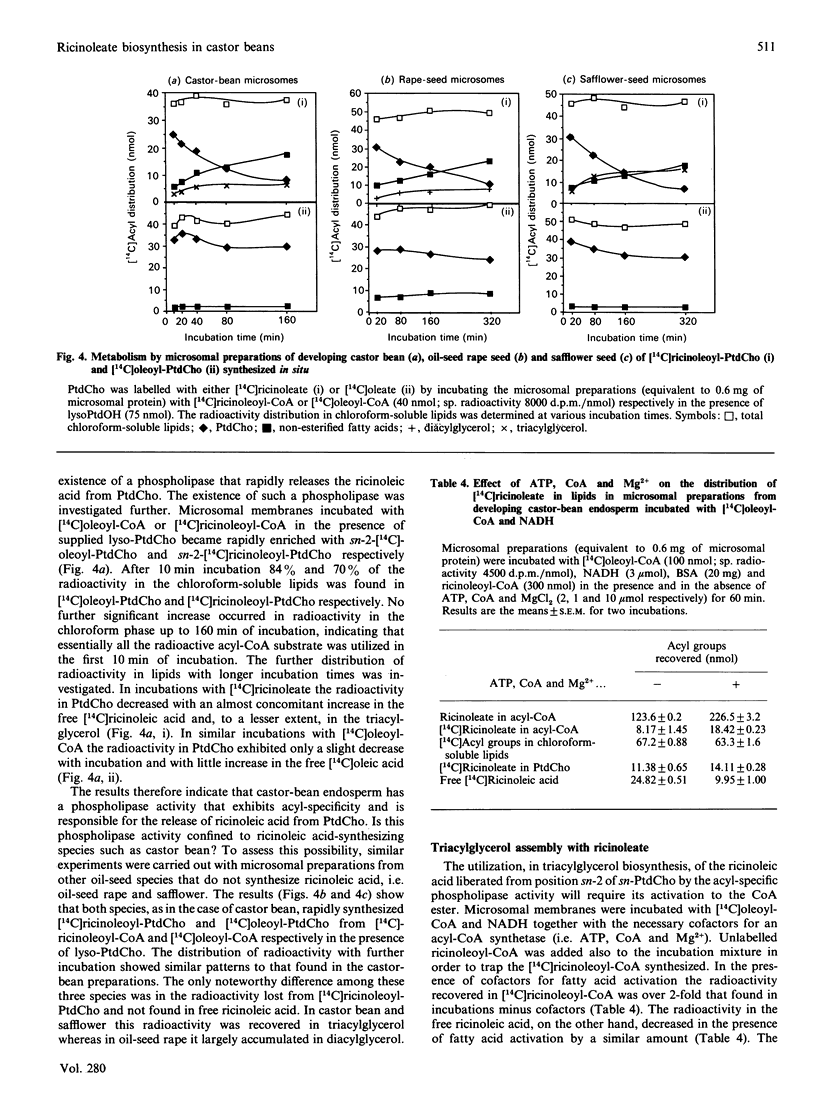
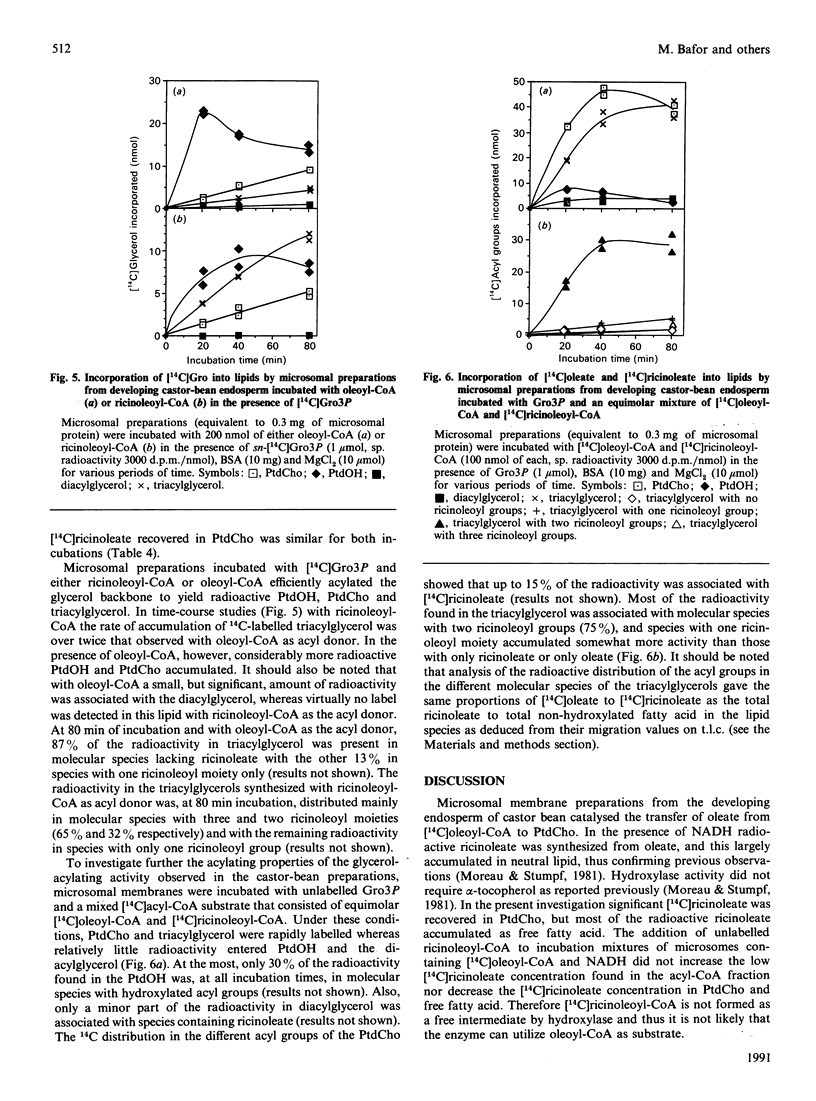
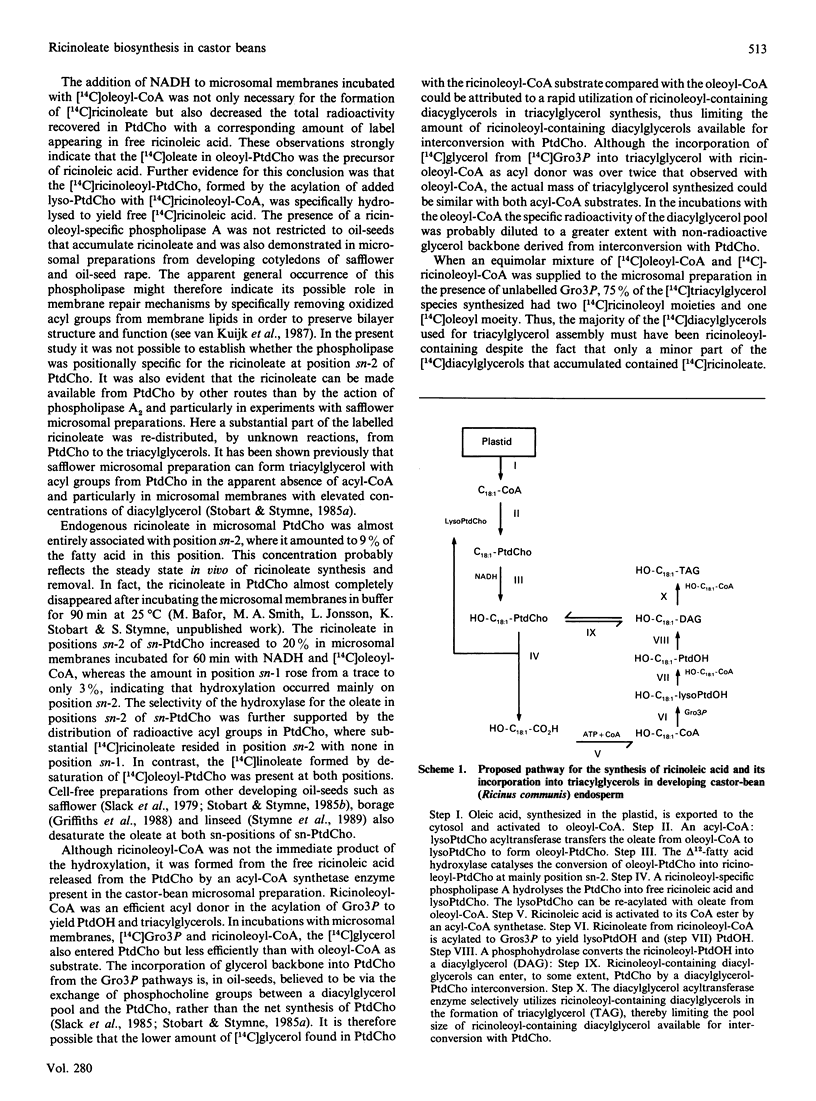
Microsomal membrane preparations from the developing endosperm of castor bean (Ricinus communis) catalysed the transfer of oleate from [14C]oleoyl-CoA to phosphatidylcholine (PtdCho). In the presence of NADH, radioactive ricinoleate (12-hydroxyoctadec-9-enoate) was synthesized from [14C]oleate, and this was largely recovered in PtdCho and as free fatty acid. The addition of unlabelled ricinoleoyl-CoA to these incubation mixtures did not increase the low [14C]ricinoleate concentration found in the acyl-CoA fraction nor decrease the [14C]ricinoleate concentration in PtdCho and free fatty acid, and thus no evidence was obtained for a hydroxylation with oleoyl-CoA as a substrate. The addition of NADH, necessary for the formation of ricinoleate, caused a decrease of the total radioactivity in PtdCho with a corresponding increase in the amount of label in free ricinoleic acid. This increase was due to the action of a phospholipase A, which released ricinoleic acid but not oleic acid from PtdCho. Such a phospholipase activity, attacking ricinoleoyl-PtdCho but not oleoyl-PtdCho, was also demonstrated in microsomal preparations from developing cotyledons of safflower and oil-seed rape. An analysis of the acyl groups at different positions in microsomal PtdCho of castor bean showed that ricinoleate was almost entirely associated with position sn-2. Likewise the [14C]ricinoleate in [14C]PtdCho formed after incubations with microsomal preparations with NADH and [14C]oleoyl-CoA resided in position sn-2 with none in position sn-1. In contrast, the [14C]linoleate formed by desaturation of [14C]oleoyl-PtdCho was present at both positions. In the presence of ATP, CoA and Mg2+, the ricinoleate acid released from PtdCho was activated to ricinoleoyl-CoA. The ricinoleoyl-CoA was an efficient acyl donor in the acylation of glycerol 3-phosphate (Gro3P) to yield phosphatidic acid and triacylglycerols. In microsomal preparations incubated with an equimolar mixture of [14C]oleoyl-CoA and [14C]ricinoleoyl-CoA in the presence of Gro3P, only a minor amount of [14C]ricinoleate entered PtdCho, and this was believed to be via the exchange of phosphocholine groups between a diacylglycerol pool and the PtdCho. On the basis of our results, a scheme of ricinoleate formation and its incorporation into triacylglycerols in castor-bean endosperm is proposed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bafor M., Jonsson L., Stobart A. K., Stymne S. Regulation of triacylglycerol biosynthesis in embryos and microsomal preparations from the developing seeds of Cuphea lanceolata. Biochem J. 1990 Nov 15;272(1):31–38. doi: 10.1042/bj2720031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliard T., Stumpf P. K. Fat metabolism in higher plants. 30. Enzymatic synthesis of ricinoleic acid by a microsomal preparation from developing Ricinus communis seeds. J Biol Chem. 1966 Dec 25;241(24):5806–5812. [PubMed] [Google Scholar]
- Griffiths G., Stobart A. K., Stymne S. Delta 6- and delta 12-desaturase activities and phosphatidic acid formation in microsomal preparations from the developing cotyledons of common borage (Borago officinalis). Biochem J. 1988 Jun 15;252(3):641–647. doi: 10.1042/bj2520641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Stobart A. K., Stymne S. The acylation of sn-glycerol 3-phosphate and the metabolism of phosphatidate in microsomal preparations from the developing cotyledons of safflower (Carthamus tinctorius L.) seed. Biochem J. 1985 Sep 1;230(2):379–388. doi: 10.1042/bj2300379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATES M. SIMPLIFIED PROCEDURES FOR HYDROLYSIS OR METHANOLYSIS OF LIPIDS. J Lipid Res. 1964 Jan;5:132–135. [PubMed] [Google Scholar]
- Moreau R. A., Stumpf P. K. Recent studies of the enzymic synthesis of ricinoleic Acid by developing castor beans. Plant Physiol. 1981 Apr;67(4):672–676. doi: 10.1104/pp.67.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Browse J. Evidence for an oleoyl phosphatidylcholine desaturase in microsomal preparations from cotyledons of safflower (Carthamus tinctorius) seed. Biochem J. 1979 Jun 1;179(3):649–656. doi: 10.1042/bj1790649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart A. K., Stymne S. The interconversion of diacylglycerol and phosphatidylcholine during triacylglycerol production in microsomal preparations of developing cotyledons of safflower (Carthamus tinctorius L.). Biochem J. 1985 Nov 15;232(1):217–221. doi: 10.1042/bj2320217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S., Stobart A. K. Evidence for the reversibility of the acyl-CoA:lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem J. 1984 Oct 15;223(2):305–314. doi: 10.1042/bj2230305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S., Stobart A. K. Involvement of acyl exchange between acyl-CoA and phosphatidylcholine in the remodelling of phosphatidylcholine in microsomal preparations of rat lung. Biochim Biophys Acta. 1985 Dec 4;837(3):239–250. doi: 10.1016/0005-2760(85)90047-5. [DOI] [PubMed] [Google Scholar]
- Sánchez M., Nicholls D. G., Brindley D. N. [The relationship between palmitoyl-coenzyme A synthetase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria]. Biochem J. 1973 Apr;132(4):697–706. doi: 10.1042/bj1320697. [DOI] [PMC free article] [PubMed] [Google Scholar]