Abstract
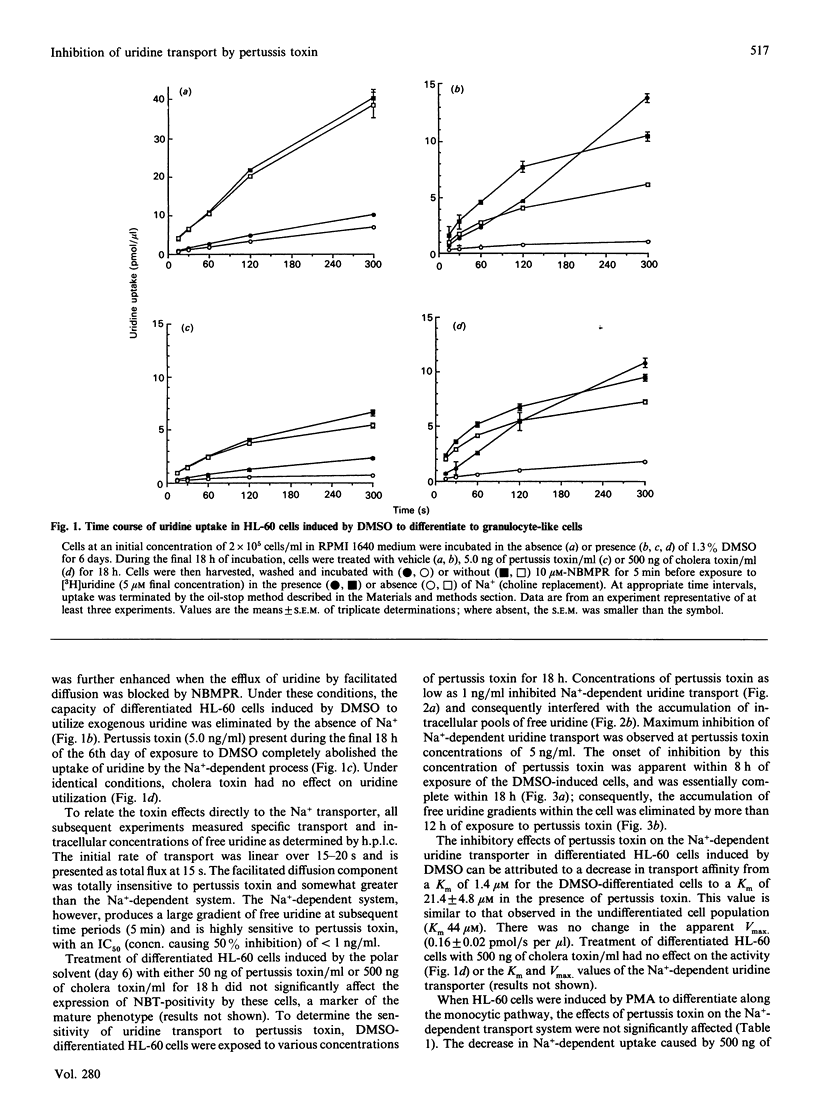
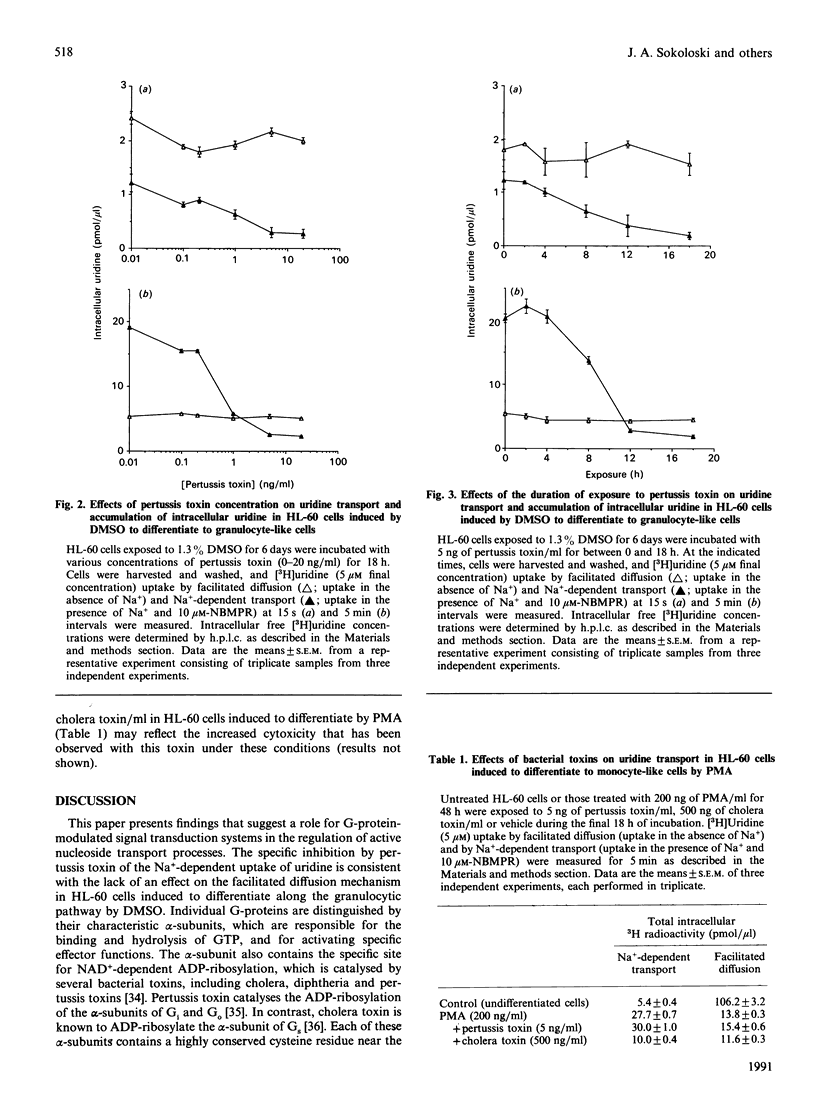
The effects of pertussis toxin on the Na(+)-dependent transport of uridine were studied in HL-60 leukaemia cells induced to differentiate along the granulocytic or monocytic pathways by dimethyl sulphoxide (DMSO) or phorbol 12-myristate 13-acetate (PMA) respectively. Pertussis toxin at 50 ng/ml completely inhibited the activation of Na(+)-dependent uridine transport and consequently prevented the formation of intracellular pools of free uridine which occurs in HL-60 cells induced to differentiate by DMSO. The inhibition of Na(+)-dependent uridine transport by pertussis toxin in cells exposed to DMSO was associated with a 14-fold decrease in affinity, with no change in Vmax. Pertussis toxin, however, had no effect on Na(+)-dependent uridine transport in PMA-induced HL-60 cells. Furthermore, 500 ng of cholera toxin/ml had no effect on the Na(+)-dependent uptake of uridine in DMSO-treated HL-60 cells. These results suggest that the activation of the Na(+)-dependent transport of uridine in HL-60 cells induced to differentiate along the granulocytic pathway by DMSO is coupled to a pertussis-toxin-sensitive guanine-nucleotide binding protein (G-protein).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Belt J. A., Noel L. D. Isolation and characterization of a mutant of L1210 murine leukemia deficient in nitrobenzylthioinosine-insensitive nucleoside transport. J Biol Chem. 1988 Sep 25;263(27):13819–13822. [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. G proteins: control of exocytosis. Nature. 1987 Jul 9;328(6126):112–113. doi: 10.1038/328112a0. [DOI] [PubMed] [Google Scholar]
- Cantiello H. F., Patenaude C. R., Ausiello D. A. G protein subunit, alpha i-3, activates a pertussis toxin-sensitive Na+ channel from the epithelial cell line, A6. J Biol Chem. 1989 Dec 15;264(35):20867–20870. [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides by human erythrocytes. Specificity toward purine nucleosides as permeants. Biochim Biophys Acta. 1973 Feb 16;291(3):734–746. doi: 10.1016/0005-2736(73)90477-x. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Accelerative exchange diffusion of uridine and thymidine and specificity toward pyrimidine nucleosides as permeants. J Biol Chem. 1972 May 25;247(10):3314–3320. [PubMed] [Google Scholar]
- Codina J., Yatani A., Grenet D., Brown A. M., Birnbaumer L. The alpha subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987 Apr 24;236(4800):442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- Darnowski J. W., Handschumacher R. E. Tissue uridine pools: evidence in vivo of a concentrative mechanism for uridine uptake. Cancer Res. 1986 Jul;46(7):3490–3494. [PubMed] [Google Scholar]
- Darnowski J. W., Holdridge C., Handschumacher R. E. Concentrative uridine transport by murine splenocytes: kinetics, substrate specificity, and sodium dependency. Cancer Res. 1987 May 15;47(10):2614–2619. [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Spiegel A., Jakobs K. H. Purification and immunochemical characterization of the major pertussis-toxin-sensitive guanine-nucleotide-binding protein of bovine-neutrophil membranes. Eur J Biochem. 1987 May 15;165(1):185–194. doi: 10.1111/j.1432-1033.1987.tb11210.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D., Cockcroft S., Howell T. W., Nüsse O., Tatham P. E. The dual effector system for exocytosis in mast cells: obligatory requirement for both Ca2+ and GTP. Biosci Rep. 1987 May;7(5):369–381. doi: 10.1007/BF01362501. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M. Characterization of sodium-dependent nucleoside transport in rabbit intestinal brush-border membrane vesicles. Biochim Biophys Acta. 1989 Feb 13;979(1):132–138. doi: 10.1016/0005-2736(89)90533-6. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside translocation in sheep reticulocytes and fetal erythrocytes: a proposed model for the nucleoside transporter. J Physiol. 1982 Mar;324:47–66. doi: 10.1113/jphysiol.1982.sp014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside transport in rat erythrocytes: two components with differences in sensitivity to inhibition by nitrobenzylthioinosine and p-chloromercuriphenyl sulfonate. J Membr Biol. 1986;93(1):1–10. doi: 10.1007/BF01871013. [DOI] [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Northup J. K., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J Biol Chem. 1984 Mar 25;259(6):3568–3577. [PubMed] [Google Scholar]
- Le Hir M., Dubach U. C. Uphill transport of pyrimidine nucleosides in renal brush border vesicles. Pflugers Arch. 1985 Jul;404(3):238–243. doi: 10.1007/BF00581245. [DOI] [PubMed] [Google Scholar]
- Lee C. W., Cheeseman C. I., Jarvis S. M. Na+- and K+-dependent uridine transport in rat renal brush-border membrane vesicles. Biochim Biophys Acta. 1988 Jul 7;942(1):139–149. doi: 10.1016/0005-2736(88)90283-0. [DOI] [PubMed] [Google Scholar]
- Lee C. W., Cheeseman C. I., Jarvis S. M. Transport characteristics of renal brush border Na(+)- and K(+)-dependent uridine carriers. Am J Physiol. 1990 May;258(5 Pt 2):F1203–F1210. doi: 10.1152/ajprenal.1990.258.5.F1203. [DOI] [PubMed] [Google Scholar]
- Lee C. W., Sokoloski J. A., Sartorelli A. C., Handschumacher R. E. Induction of the differentiation of HL-60 cells by phorbol 12-myristate 13-acetate activates a Na(+)-dependent uridine-transport system. Involvement of protein kinase C. Biochem J. 1991 Feb 15;274(Pt 1):85–90. doi: 10.1042/bj2740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Nasmith P. E., Mills G. B., Grinstein S. Guanine nucleotides induce tyrosine phosphorylation and activation of the respiratory burst in neutrophils. Biochem J. 1989 Feb 1;257(3):893–897. doi: 10.1042/bj2570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M. Nucleoside transport in cultured mammalian cells. Multiple forms with different sensitivity to inhibition by nitrobenzylthioinosine or hypoxanthine. Biochim Biophys Acta. 1984 Jun 13;773(1):39–52. doi: 10.1016/0005-2736(84)90548-0. [DOI] [PubMed] [Google Scholar]
- Schwenk M., Hegazy E., Lopez del Pino V. Uridine uptake by isolated intestinal epithelial cells of guinea pig. Biochim Biophys Acta. 1984 Dec 11;805(4):370–374. doi: 10.1016/0167-4889(84)90020-x. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Cox C. C., Snyderman R. Receptor-coupled activation of phosphoinositide-specific phospholipase C by an N protein. Science. 1986 Apr 4;232(4746):97–100. doi: 10.1126/science.3006254. [DOI] [PubMed] [Google Scholar]
- Sokoloski J. A., Blair O. C., Sartorelli A. C. Alterations in glycoprotein synthesis and guanosine triphosphate levels associated with the differentiation of HL-60 leukemia cells produced by inhibitors of inosine 5'-phosphate dehydrogenase. Cancer Res. 1986 May;46(5):2314–2319. [PubMed] [Google Scholar]
- Spiegel A. M., Brown E. M., Fedak S. A., Woodard C. J., Aurbach G. D. Holocatalytic state of adenylate cyclase in turkey erythrocyte membranes: formation with guanylylimidodiphosphate plus isoproterenol without effect on affinity of beta-receptor. J Cyclic Nucleotide Res. 1976;2(1):47–56. [PubMed] [Google Scholar]
- Trimble M. E., Coulson R. Adenosine transport in perfused rat kidney and renal cortical membrane vesicles. Am J Physiol. 1984 Jun;246(6 Pt 2):F794–F803. doi: 10.1152/ajprenal.1984.246.6.F794. [DOI] [PubMed] [Google Scholar]
- West R. E., Jr, Moss J., Vaughan M., Liu T., Liu T. Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985 Nov 25;260(27):14428–14430. [PubMed] [Google Scholar]
- Williams T. C., Jarvis S. M. Multiple sodium-dependent nucleoside transport systems in bovine renal brush-border membrane vesicles. Biochem J. 1991 Feb 15;274(Pt 1):27–33. doi: 10.1042/bj2740027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M. Nucleoside transport in animal cells. Biosci Rep. 1983 Apr;3(4):309–322. doi: 10.1007/BF01122895. [DOI] [PubMed] [Google Scholar]


