Abstract
Recent advancements in Alzheimer’s disease treatment have focused on the elimination of amyloid-beta (Aβ) plaque, a hallmark of the disease. Monoclonal antibodies such as lecanemab and donanemab can alter disease progression by binding to different forms of Aβ aggregates. However, these treatments raise concerns about adverse effects, particularly amyloid-related imaging abnormalities (ARIA). Careful assessment of safety, especially regarding ARIA, is crucial. ARIA results from treatment-related disruption of vascular integrity and increased vascular permeability, leading to the leakage of proteinaceous fluid (ARIA-E) and heme products (ARIA-H). ARIA-E indicates treatment-induced edema or sulcal effusion, while ARIA-H indicates treatment-induced microhemorrhage or superficial siderosis. The minimum recommended magnetic resonance imaging sequences for ARIA assessment are T2-FLAIR, T2* gradient echo (GRE), and diffusion-weighted imaging (DWI). T2-FLAIR and T2* GRE are necessary to detect ARIA-E and ARIA-H, respectively. DWI plays a role in differentiating ARIA-E from acute to subacute infarcts. Physicians, including radiologists, must be familiar with the imaging features of ARIA, the appropriate imaging protocol for the ARIA workup, and the reporting of findings in clinical practice. This review aims to describe the clinical and imaging features of ARIA and suggest points for the timely detection and monitoring of ARIA in clinical practice.
Keywords: Amyloid-related imaging abnormalities, Monoclonal antibodies, Magnetic resonance imaging
INTRODUCTION
Recent advancements in Alzheimer’s disease (AD) treatment have focused on eliminating amyloid-beta (Aβ) plaques, which are a characteristic feature of the disease [1]. The current period is characterized by the development of monoclonal antibodies, including lecanemab and donanemab, which have shown the potential to alter disease progression [1]. Concerning target specificity, solanezumab, crenezumab, and other first-generation monoclonal antibodies primarily bind to amyloid monomers and soluble small aggregates; contrarily, second-generation antibodies such as aducanumab, lecanemab, donanemab, and gantenerumab have a higher binding affinity for pathogenic amyloid aggregates like oligomers, protofibrils, and fibrils [2]. Consequently, second-generation monoclonal antibodies demonstrate enhanced clinical efficacy and faster Aβ clearance than their first-generation counterparts [1]. However, this enhanced amyloid-beta clearance is accompanied by a greater risk of adverse effects, such as amyloid-related imaging abnormalities (ARIA), compared to first-generation monoclonal antibodies [3].
The advent of aducanumab, lecanemab, and donanemab represents a significant achievement in the treatment of AD. The primary concern is the potential occurrence of vascular side effects known as ARIA, including ARIA-E (edema or effusion) and ARIA-H (microhemorrhages and hemosiderosis) [4]. The safety profiles for ARIA should be navigated with caution. Further research is essential to ascertain the optimal application and long-term effects of these innovative therapeutic agents in the management of AD. This review aims to describe the clinical and imaging features of ARIA and suggest points for timely detection and monitoring of ARIA in clinical practice.
Anti-Amyloid Beta Monoclonal Antibodies for the Treatment of Alzheimer’s Disease
Aducanumab, a human-derived antibody, explicitly targets aggregated forms of Aβ that accumulate in the brain, including soluble oligomers and fibrils [5]. In clinical trials, aducanumab has been shown to reduce Aβ-related and downstream biomarkers in patients with AD effectively [6]. However, its clinical efficacy has been controversial. The FDA conditionally approved aducanumab as the first anti-Aβ immunotherapy agent, stating that the substantial amyloid-lowering effects were considered reasonably likely to confer a clinical benefit [7]. However, the development and commercialization of aducanumab have been discontinued, and the phase 4 ENVISION study was terminated. This decision was not due to efficacy or safety issues but rather to reallocate funding and focus on lecanemab, the second FDA-approved anti-Aβ immunotherapy agent.
Lecanemab is a humanized IgG1 monoclonal antibody that specifically targets Aβ protofibrils [8]. Its primary mechanism of action is to reduce pathogenic Aβ levels, inhibit Aβ deposition, and specifically diminish Aβ protofibrils in the brain and cerebrospinal fluid (CSF), as demonstrated in animal models of AD [9]. In the Clarity AD trial, lecanemab was shown to slow the progression of cognitive and functional decline in patients with early-stage AD, with improvements in CSF and plasma biomarkers, including Aβ and phosphorylated tau, leading to subsequent full FDA approval [10]. The AHEAD 3-45 trial (NCT04468659) is also underway to determine the efficacy and safety of lecanemab in patients with preclinical AD. Considering the risk factors of ARIA, as explained later, the inclusion and exclusion criteria for the use of lecanemab were determined. Among the detailed items in the criteria of Appropriate Use Recommendations of lecanemab, the clinically critical and relevant radiologic criteria are shown in Table 1 [11].
Table 1. The clinically critical and relevant to radiologic criteria of Appropriate Use Recommendations of lecanemab.
| Criteria of Appropriate Use Recommendations of Lecanemab | |
|---|---|
| Clinical diagnosis of MCI or mild AD dementia | |
| Positive amyloid PET or CSF studies | |
| MMSE 22–30 or other cognitive screening instrument with a score compatible with early AD | |
| Exclusion criteria: | |
| A) Any medical, neurologic, or psychiatric condition that may be contributing to the cognitive impairment or any non-AD MCI or dementia | |
| B) More than 4 microhemorrhages | |
| C) A single macrohemorrhage >10 mm at the greatest diameter | |
| D) An area of superficial siderosis | |
| E) Evidence of vasogenic edema | |
| F) More than 2 lacunar infarcts or a stroke involving a major vascular territory | |
| G) Severe subcortical hyperintensities consistent with a Fazekas score of 3 | |
| H) Evidence of amyloid beta-related angiitis or CAA-related inflammation | |
| I) Or other major intracranial pathology that may cause cognitive impairment | |
| J) MRI evidence of a non-AD dementia | |
| K) Patients on anticoagulants should not receive lecanemab; tPA should not be administered to individuals on lecanemab | |
| L) Unstable medical conditions that may affect or be affected by lecanemab therapy, including mental illness, major depression, recent history of stroke, and coagulopathy | |
MCI = mild cognitive impairment, AD = Alzheimer’s disease, CSF = cerebrospinal fluid, MMSE = mini-mental state examination, CAA = cerebral amyloid angiopathy, tPA = tissue plasminogen activator
Donanemab targets a particular variant of Aβ species, specifically a truncated and N-terminally modified pyroglutamate-3 Aβ epitope (N3pG). This precise targeting makes it possible to achieve more efficient removal and prevention of amyloid plaques, as N3pG Aβ is hypothesized to act as a seed for plaque deposition [12]. The TRAILBLAZER-ALZ2 trials indicated that donanemab successfully mitigated cognitive and functional deterioration in AD [13]. This approach could provide a novel avenue for the treatment of AD, specifically during its initial phases.
Basics of Amyloid-Related Imaging Abnormalities
Definition
In 2010, the Alzheimer’s Research Roundtable convened a workgroup to provide information and recommendations regarding imaging abnormalities encountered in anti-amyloid trials [14]. This workgroup termed ARIA and refined the terminology: ARIA-E for edema or effusion and ARIA-H for microhemorrhages and hemosiderosis. ARIA-E refers to vasogenic edema in the brain parenchyma or sulcal effusions (Figs. 1, 2, 3, 4, 5, 6). Vasogenic edema is visualized as an increased MR signal intensity on FLAIR sequences that is typically transient and is not associated with evidence of restricted diffusion, tissue necrosis, or other sequelae associated with cytotoxic edema. The term ‘ARIA-H’ refers to hemosiderin deposits presenting as microhemorrhages (hemorrhages <10 mm) in the brain parenchyma and/or localized superficial siderosis (located in the subarachnoid space) (Figs. 3, 5, 6). ARIA-H is detected using heme-sensitive sequences, such as T2* gradient echo (GRE) and susceptibility-weighted imaging (SWI).
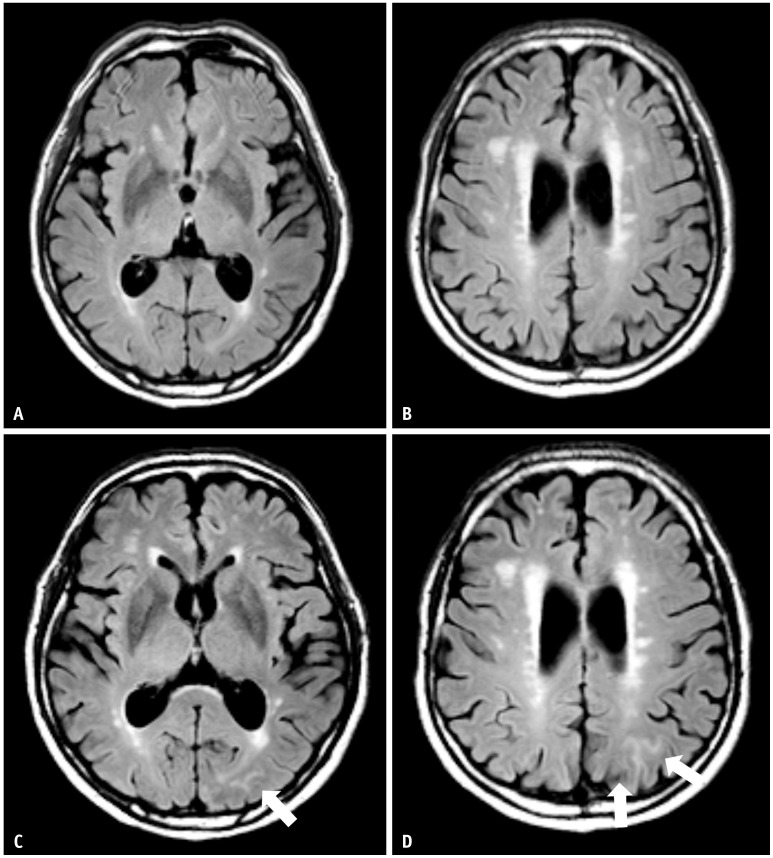
Fig. 1. A 75-year-old male patient with mild cognitive impairment presented with transient ARIA-E during gantenerumab therapy. A, B: His baseline MRI shows a moderate degree of white matter hyperintensity. C, D: Nine weeks later, a surveillance MRI showed sulcal FLAIR with high signal intensity in the left parietal area, suggesting ARIA-E (arrows). The patient was asymptomatic. Since a single region and extent measuring less than 5 cm would be classified as mild, this was an asymptomatic and mild case, and the gantenerumab was continuously taken. At follow-up surveillance MRI after initial ARIA-E detection, it was completely resolved.
Fig. 2. A 74-year-old female patient with mild cognitive impairment presented with severe ARIA-E during lecanemab (BAN-2401) therapy. A, B: Her baseline MRI shows a mild degree of white matter hyperintensity. C, D: Eight weeks later, she had a headache and confusion. MRI shows multifocal FLAIR high signal intensity parenchymal edema with swelling in the bilateral cerebral hemispheres, suggestive of ARIA-E (arrows). Multiple lesions with an extent measuring more than 10 cm were present and classified as severe. The lecanemab therapy was discontinued. The extent of ARIA-E gradually decreased on follow-up MRI and completely resolved at the follow-up surveillance MRI after the initial ARIA-E detection.
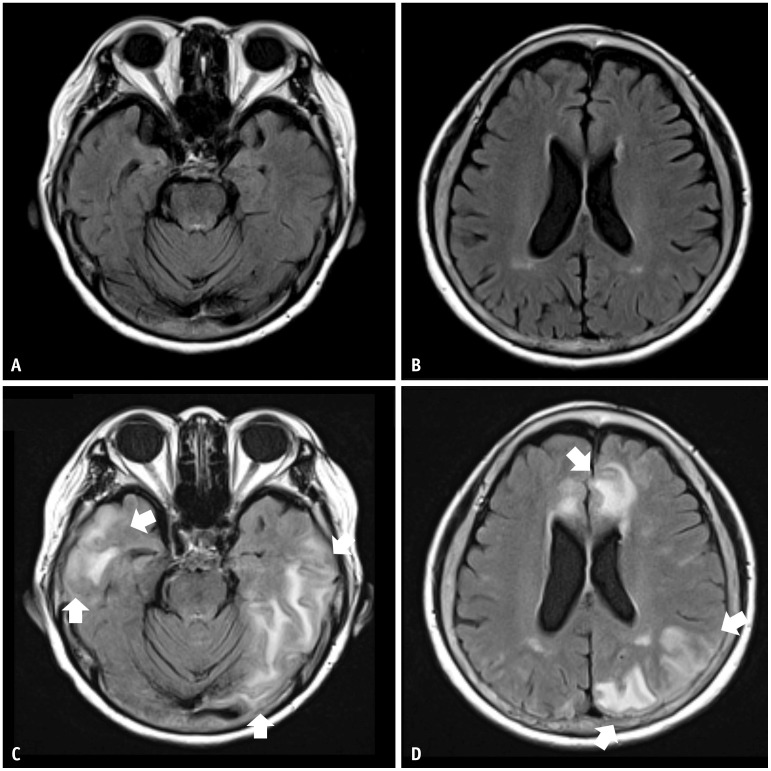
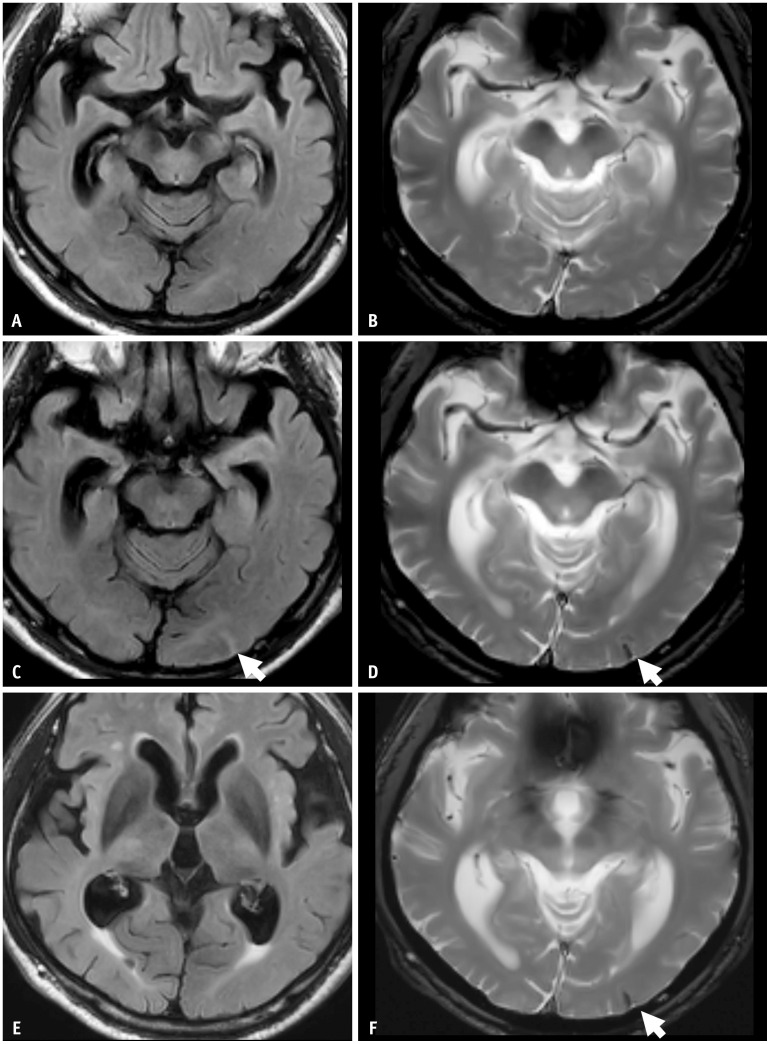
Fig. 3. An 80-year-old female patient with mild cognitive impairment concurrently presented with both ARIA-E and ARIA-H during aducanumab therapy. She was an APOE-ε4 homozygote carrier. A-D: Her baseline MRI shows moderate white matter hyperintensity but no microbleeds. Five months later, she complained of a headache and confusion. E-H: Multifocal FLAIR high signal intensity edema in both cerebral hemispheres showed multiple microbleeds, suggestive of ARIA-E and ARIA-H on follow-up MRI. The MRI showed multifocal FLAIR high signal intensity edema (arrows in E, F) and multiple microbleeds (arrows in G, H) in both cerebral hemispheres, suggestive of ARIA-E and ARIA-H. She was discontinued from the lecanemab therapy, and her symptoms gradually resolved. Three months after the infusion suspension, ARIA-E was resolved, and ARIA-H was stabilized on a follow-up MRI.
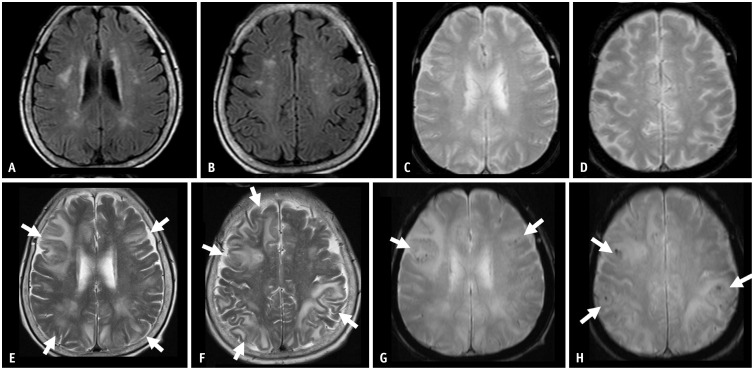
Fig. 4. A 69-year-old female patient presented with mild cognitive impairment and was treated with gantenerumab therapy. A, B: Her baseline MRI shows a mild degree of white matter hyperintensity. C, D: Seven months after the initiation of gantenerumab therapy, the patient had an episode of syncope, and the MRI showed multifocal sulcal FLAIR high-signal intensity in the right parietal lobe, suggesting ARIA-E (arrows). The case had two regions (arrows) with an extent measuring between 5 cm and 10 cm, classified as moderate. As a symptomatic and moderate case, the gantenerumab therapy was suspended. ARIA-E was resolved after suspending treatment for 1 month.
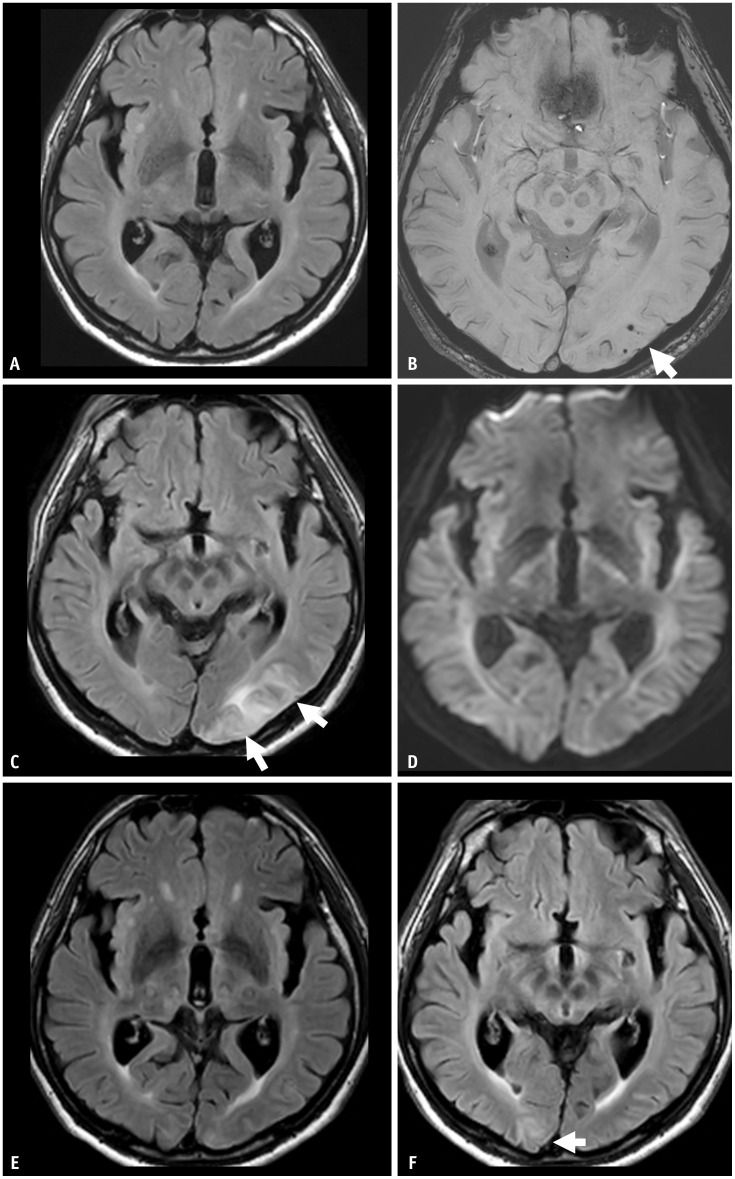
Fig. 5. A 67-year-old male patient presented with mild cognitive impairment and was treated with lecanemab therapy. He was an APOE-ε4 homozygote carrier. A, B: His baseline MRI shows a mild degree of white matter hyperintensity and three microbleeds (arrow) in the left occipital lobe. Eleven months after initiation of the lecanemab therapy, the patient complained of a headache. C, D: MRI shows sulcal and parenchymal FLAIR high-signal intensity without diffusion restriction in the left occipital lobe, suggesting ARIA-E (arrows). A single region with an extent measuring between 5 cm and 10 cm was classified as moderate. As a symptomatic and moderate case, the lecanemab therapy was suspended. E: On follow-up MRI after suspending treatment for 2 months, ARIA-E was resolved. After the resolution of ARIA-E, the lecanemab treatment was resumed. F: Two months after the resumption of the therapy, a new sulcal and parenchymal FLAIR high-signal intensity (arrow) appeared in the right occipital lobe, and the patient permanently suspended the treatment.
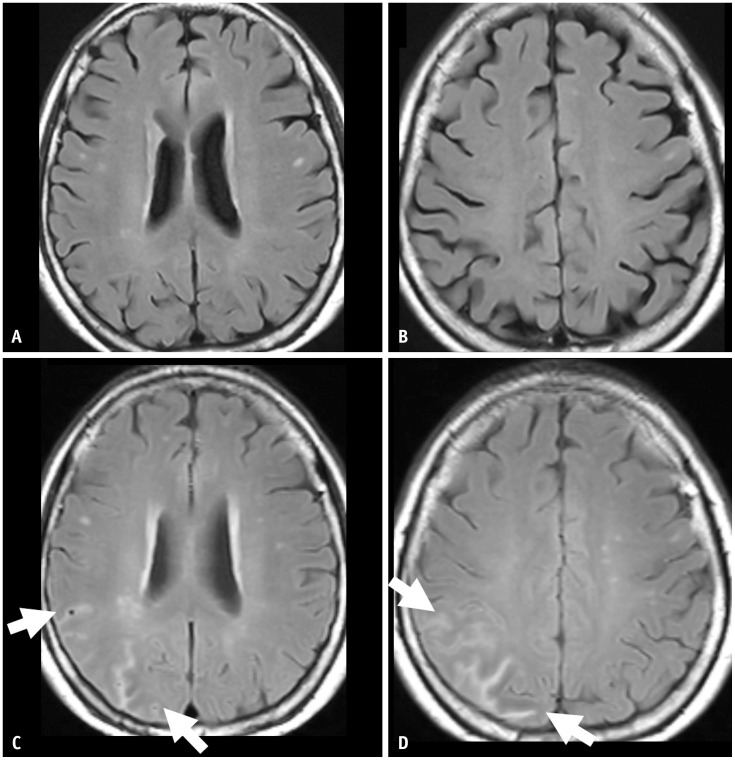
Fig. 6. A 68-year-old male patient presented with mild cognitive impairment and was treated with lecanemab therapy. He was an APOE-ε3/ε4 heterozygote carrier. A, B: His baseline MRI shows no abnormalities except bilateral medial temporal lobe atrophy. C, D: On the surveillance MRI performed 6 months after the lecanemab therapy, there was focal sulcal FLAIR high-signal intensity effusion and superficial siderosis on gradient echo sequences in the left occipital lobe, suggestive of ARIA-E and ARIA-H (arrows). Both the sulcal effusion and superficial siderosis were single lesions with an extent less than 5 cm, classified as mild ARIA-E and ARIA-H. As an asymptomatic mild ARIA case, lecanemab therapy was continued. E, F: On a 1-month follow-up MRI after the ARIA event, the ARIA-E was resolved, and the ARIA-H (arrow) remained stable.
Pathophysiology of ARIA
ARIA is a phenomenon primarily diagnosed based on imaging, and its precise pathophysiological correlates remain partially understood. During AD, cerebral vascular structures evolve from normal to AD-like vascular pathology, which is associated with vascular amyloid deposition, disrupted vascular integrity, and impaired perivascular clearance pathways [15,16,17]. Age and the presence of the APOE-ε4 genotype may contribute to pre-existing amyloid vascular pathology states. It is hypothesized that when immunotherapy is initiated, monoclonal antibodies bind to accumulated Aβ in the cerebral parenchyma and cerebral vessels [18,19]. Bound monoclonal antibodies mobilize large amounts of amyloid through the perivascular drainage pathway [14,20]. The mobilization of amyloid plaques in blood vessels causes periarterial inflammation, resulting in swelling and microhemorrhages, which are detected on MRI. This mobilization of amyloid plaques and adjacent inflammation disrupt vascular integrity and increased vascular permeability, leading to the leakage of proteinaceous fluid (ARIA-E) and heme products (ARIA-H).
ARIA and cerebral amyloid angiopathy-related inflammation (CAA-ri) may share the exact pathophysiology. In the acute or subacute stage of CAA-ri, anti-Aβ autoantibodies in CSF are elevated and return to the typically observed level in patients with AD and noninflammatory CAA after clinicopathologic resolution [21,22]. Based on this evidence, ARIA-E resulting from immunotherapy in AD is increasingly recognized as an iatrogenic manifestation of CAA-ri, which is the spontaneous ARIA-E associated with increased CSF anti-Aβ autoantibodies occurring in both AD and CAA patients. Solopova et al. [23] reported an autopsy case of a fatal ARIA that occurred during lecanemab treatment of AD. The postmortem MRI and neuropathological features of severe ARIA were acute arteritis, resembling the sporadic condition of CAA-ri. Pathologically, the condition was associated with marked perivascular inflammation and arteriolar degeneration, leading to microhemorrhagic changes in both the parenchyma and leptomeninges. The inflammatory features include extensive macrophages and activated microglia in the leptomeninges, perivascular space, and parenchyma, which were associated with severe CAA [19,23]. A study by Piazza et al. [22] demonstrated a direct association between neuroinflammation and CAA-ri in patients with high CSF anti-Aβ antibody levels and microglial activation, as shown by 11C-PK11195 PET.
Approximately half of the ARIA-E cases are accompanied by ARIA-H, primarily cortical microbleeds [24]. Similar to the case shown in Figure 3, microbleeds tend to persist or somewhat increase even after ARIA-E resolution. This may have implications for the possible pathomechanisms reminiscent of CAA.
Incidence of ARIA
Meta-analyses of clinical trials involving patients treated with anti-Aβ immunotherapy reported overall incidences of ARIA-E and ARIA-H at 6.5% and 7.8%, respectively [3]. In a subgroup analysis, the incidence of ARIA varied according to the specific anti-Aβ immunotherapy agent and APOE-ε4 carrier status [3]. A recent phase 3 clinical trial of lecanemab reported overall incidences of ARIA-E, ARIA-H, and any ARIA (including both APOE-ε4 allele carrier and non-carrier) as 12.6%, 17.3%, and 21.5%, respectively [10]. For donanemab, the reported incidences of ARIA-E, ARIA-H, and any ARIA (including both APOE-ε4 allele carrier and non-carrier) were 24.0%, 31.4%, and 36.8%, respectively [13].
Risk Factors of ARIA
Various risk factors of ARIA have been reported in clinical trials of anti-Aβ immunotherapy [6,14,25,26]. Commonly identified risk factors in clinical trials include drug exposure, APOE-ε4 allele carrier status, and pretreatment microhemorrhages [6,14,18,25]. Regarding drug exposure, ARIA occurs more frequently at higher drug doses and earlier in the treatment course of anti-Aβ immunotherapy. Higher drug doses and early treatment increase perivascular Aβ clearance, leading to greater vascular permeability and the extravasation of proteinaceous fluid and erythrocytes, resulting in ARIA-E and ARIA-H, respectively. Therefore, the risk of developing ARIA can be reduced if patients start at a low dose, with progressive titration to a higher therapeutic dose over time. This gradual adjustment period allows the cerebral vessels to smoothly undergo the transient process of structural integrity challenge caused by the removal of amyloid deposits. Consequently, this extended timeline enables the restoration of vascular integrity when the patient reaches higher treatment doses. Dose titration and escalation strategies have become commonplace in anti-amyloid monoclonal antibody treatment studies. For example, in the phase III trials of aducanumab, the rates of ARIA were lower in the dose-titration group compared to the non-titration group [10]. In addition, in phase III clinical trials of donanemab, most of the first ARIA cases occurred after receiving 1 to 3 donanemab infusions [13].
APOE-ε4 carrier status is the most significant risk factor for the development of ARIA besides drug dose. APOE-ε4 carriers will likely have higher amyloid deposition levels in their vessel walls. In various clinical trials, the incidence of ARIA was highest in homozygous APOE-ε4 carriers, followed by heterozygous carriers, and lowest in non-carriers. In the bapineuzumab studies, patients with the heterozygotes APOE-ε4 had a hazard ratio of 4.1 for developing ARIA-H, while with homozygotes APOE-ε4 alleles had a hazard ratio of 12.8 [27]. In lecanemab studies, the incidence of ARIA-H was increased with APOE-ε4 carrier status with the incidence of 39.0%, 14.0%, and 11.9% in homozygotes APOE-ε4, heterozygotes APOE-ε4, and non-carrier, respectively [10]. In a meta-analysis, the incidence of ARIA was also higher in the APOE-ε4 carrier group than that of the APOE-ε4 non-carrier group (8.6% [3.9%–17.9%] vs. 6.9% [3.7%–12.5%]) [3]. Given this, increased risk for ARIA among individuals with homozygous APOE-ε4 status, APOE genotyping before the initiation of drug therapy could determine the frequency of safety monitoring evaluations in future treatment guidelines.
Patients with preexisting hemosiderin deposits, particularly lobar microhemorrhages and superficial siderosis indicative of underlying CAA, have a significantly higher risk of ARIA, especially in individuals carrying APOE-ε4 [4,28]. Many studies have shown that ARIA shares similarities with CAA-ri in imaging findings, pathology, and response to treatment outcomes [19,23]. ARIA-E induces the iatrogenic manifestations of CAA-ri. Pretreatment microhemorrhages, antibody binding sites, targeted antibody structures, and genetic factors promoting AD pathology, such as Down syndrome and PSEN1, PSEN2, and APP mutations, are suggested as risk factors [29]. In the aducanumab and lecanemab trials, patients with more than four microhemorrhages were excluded due to the increased risk of ARIA-E and ARIA-H levels [11,30].
Clinical Presentation of ARIA
Most cases of ARIA during anti-Aβ therapies tend to be asymptomatic. Symptomatic cases have been reported in 6.1% to 39.3% of cases, depending on the different agents and therapeutic doses [10,13,23,31]. In a previous meta-analysis, the pooled incidence of asymptomatic ARIA was 80.4% [3]. Most patients with symptomatic ARIA present with transient and mild, nonspecific symptoms. The most commonly reported symptoms are headache, confusion, vomiting, and visual or gait disturbance [10,13,14,18,23,31,32,33]. However, severe reactions, such as brain swelling, seizures, and death, have been reported with ARIA. In a lecanemab trial, 22.1% of patients who developed ARIA-E and 0.4% of patients who developed ARIA-H presented with symptoms [10].
Radiological Identification of ARIA
Radiologic Severity
In the management of ARIA, the symptoms and radiographic severity of ARIA determine whether treatment should be continued, modified, or suspended. Therefore, a quantitative and objective evaluation of the severity of ARIA is necessary. Attempts have been made to define severity scales that can guide physicians in determining dose adjustments or suspensions. Several radiological severity grading schemes have been proposed to quantify ARIA, including the Barkhof Grand Total Scale and the 3- and 5-point Severity Scales of ARIA-E (SSAE-3 and SSAE-5) [27,34,35].
The FDA guidelines define mild, moderate, and severe ARIA-E and ARIA-H (Table 2) [36]. The severity of ARIA-E depends on the location and extent of abnormalities. A FLAIR hyperintensity in a single location that is less than 5 cm was graded as mild (Figs. 1, 6). FLAIR hyperintensity measurements between 5 and 10 cm, or those observed in multiple locations, were graded as moderate (Figs. 4, 5). Any FLAIR hyperintensity greater than 10 cm was graded as severe (Figs. 2, 3).
Table 2. Radiographic severity of ARIA.
| ARIA type | Mild | Moderate | Severe |
|---|---|---|---|
| ARIA-E | FLAIR hyperintensity confined to sulcus and/or cortex/subcortex white matter in one location <5 cm | FLAIR hyperintensity 5 to 10 cm in single greatest dimension, or more than 1 site of involvement, each measuring <10 cm | FLAIR hyperintensity >10 cm with associated gyral swelling and sulcal effacement. One or more separate/independent sites of involvement may be noted |
| ARIA-H, microhemorrhage | ≤4 new incident microhemorrhages | 5 to 9 new incident microhemorrhages | 10 or more new incident microhemorrhages |
| ARIA-H, superficial siderosis | 1 focal area of superficial siderosis | 2 focal areas of superficial siderosis | >2 areas of superficial siderosis |
ARIA = amyloid-related imaging abnormalities
Regarding ARIA-H, severity was graded according to the number of microhemorrhages or focal areas of superficial siderosis. Fewer than four microhemorrhages or one area of superficial siderosis was graded as mild (Fig. 6), 5–9 microhemorrhages or two regions of superficial siderosis as moderate, and more than ten microhemorrhages or more than two areas of superficial siderosis as severe (Fig. 3).
The Barkhof Grand Total Scale is a 60-point rating system that assesses the ARIA-E in six regions (frontal, parietal, occipital, temporal, central, and infratentorial) of the brain on both the left and right sides, yielding 12 separate anatomic locations for evaluation. At each location, the lesions are characterized as parenchymal hyperintensity, sulcal hyperintensity, and swelling and then scored accordingly [35]. The SSAE-3 and SSAE-5 are simpler severity grading systems of ARIA-E, based on a single linear measurement of the largest area of the lesion (<5 cm, 5 to 10 cm, or >10 cm) and multiplicity [37]. In a validation study, both the SSAE-3 and SSAE-5 are equally valid with good inter- and intra-reader agreement. However, in real clinical practice, the 5-point scale (SSAE-5) allows for more flexibility in treatment management and may be preferred for managing patients with small multi-focal ARIA-E presentations [38].
MRI Protocol
The Alzheimer’s Association Research Roundtable working group suggested a minimum standard MRI protocol [14], which can be implemented in a wide variety of care settings, particularly in an average community-based setting, as follows: 1) Scanner field strength: the use of 1.5T scanners is endorsed as a minimum standard, 2) Scan sequences: Two-dimensional (2D) T2* GRE to identify ARIA-H is presently available on any scanner worldwide and is recommended, 3) Slice thickness of 5 mm or less, 4) echo time (TE) = 20 ms or greater, and 5) T2 FLAIR sequence for the identification of ARIA-E (Table 3) [14].
Table 3. MRI protocol for ARIA.
| Component | MRI protocol |
|---|---|
| Scanner field strength | 1.5T (minimum) or 3T (recommended) |
| Slice thickness | ≤5 mm |
| Echo time | ≥20 ms |
| Scan time | ≤15 min (recommended) |
| Imaging sequence | T2-FLAIR (2D or 3D): For ARIA-E (vasogenic edema in the parenchyma, effusion and exudate in the leptomeninges) |
| T2* GRE (or SWI)*: For ARIA-H (microhemorrhage in the parenchyma, superficial siderosis in the leptomeninges) | |
| Diffusion-weighted imaging: For the differential diagnosis of cytotoxic edema including acute infarction | |
| 3D T1-imaging (optional): For the assessment of brain atrophy and disease progression |
*The sequence of the baseline and follow up monitoring MRI (T2* GRE or SWI) should be consistent and matching.
ARIA = amyloid-related imaging abnormalities, D = dimensional, GRE = gradient echo, SWI = susceptibility-weighted imaging
The minimum recommended sequences for ARIA assessment are T2-FLAIR, T2* GRE, and DWI. T2-FLAIR imaging is necessary for ARIA-E detection. 2D axial FLAIR or 3D FLAIR can be performed using reliable, reproducible techniques that can be used in clinical practice. The 2D axial T2-FLAIR imaging has been performed in early clinical trials. Over the past decade, 3D FLAIR imaging has been shown to have the advantages of improved CSF suppression and increased sensitivity for parenchymal edema, and it has become more widely performed [39,40]. Therefore, 3D FLAIR may be preferred if feasible and can be performed routinely with high quality and standardization.
T2* sequences are required for ARIA-H assessment. Theoretically, the SWI sequence, which is more sensitive to local field inhomogeneities (hemorrhages) than the T2* sequence, is ideal for the detection and evaluation of ARIA-H. However, in real-world clinical practice, diverse MR vendors and the unavailability of uniform sequences across institutions should be considered. Additionally, since the current guidelines for anti-Aβ immunotherapy agents do not specify the number of microbleeds based on SWI sequence, future research to clarify these standards is necessary. Until a uniform standard for a GRE scan is established, it is important to ensure consistency and matching of the T2* sequence of baseline and follow-up MRI monitoring.
The DWI sequence plays an important role in differentiating ARIA-E from potential cytotoxic edema, as may be noted with an incidental acute-to-subacute infarct (Fig. 5). Therefore, DWI is recommended as a routine protocol.
In treatment with aducanumab, lecanemab, or donanemab, acceleration of brain volume loss has been reported, which is attributed to amyloid removal [41,42,43]. Therefore, 3D T1-weighted anatomical imaging is recommended for evaluating brain volume.
Baseline (Pretreatment) MRI
Before initiation of anti-Aβ immunotherapy, brain MRI with standardized basic sequences (T2-FLAIR, T2* GRE, DWI) is required to evaluate the patient for pre-existing microhemorrhages or hemosiderin deposition associated with an increased risk of ARIA [44,45]. In addition, these MRI scans provide a baseline for comparison during subsequent safety monitoring and follow-up examinations. In the Appropriate Use Recommendations for Lecanemab, baseline MRI should be performed within 12 months before initiating treatment [11]. However, in real-world clinical settings, a 12-month interval may allow disease progression, stroke, and occurrences of microbleeds. Therefore, it is advisable to conduct a baseline MRI immediately before starting treatment. Consistency in MRI acquisition is important for the accurate diagnosis of ARIA, not only with the field strength but also with the vendor type.
In the clinical trials, the exclusion criteria were ≥5 microhemorrhages or any superficial siderosis on the baseline MR imaging. The recent FDA guidelines for the clinical use of aducanumab suggest exclusion criteria of >4 microhemorrhages, any area of superficial siderosis, or parenchymal hemorrhages measuring >1 cm in the previous year [30]. The FDA guidelines for the clinical use of lecanemab also suggest the same exclusion criteria for hemorrhage [11]. Baseline imaging should be performed using the same MR protocol as that will be used for subsequent safety monitoring examinations.
Monitoring MRI
Frequent monitoring of all ARIA-Es was not feasible. In addition, the clinical effect of “missing” an asymptomatic, transient ARIA-E is still unclear. In addition to scheduled surveillance scans, scans should be prompted by the onset of symptoms suggestive of ARIA-E. Symptoms of ARIA may be nonspecific and include headache, vomiting, nausea, confusion, dizziness, visual disturbance, gait difficulties, loss of coordination, tremor, transient ischemic attack, new-onset seizures, and significant and unexpected acute cognitive decline. Combined surveillance and interventional MRI should adequately characterize the incidence and evaluation of ARIA.
The appropriate use of lecanemab requires MRIs before the 5th, 7th, and 14th infusions [11]. Furthermore, an additional MRI is recommended at week 52 (i.e., before the 26th infusion, especially for APOE-ε4 carriers and those with evidence of ARIA on earlier MRIs). These scheduled monitoring sessions should be supplemented by intervening safety MRI scans obtained from patients with symptoms suspected to be caused by ARIA [11]. A monthly MRI should be performed until ARIA-E resolves or ARIA-H stabilizes. In addition to these scheduled MRI scans, patients should also undergo MRI whenever they experience symptoms that may be related to ARIA [26].
In AD, brain volume loss and increased ventricular size have been consistently associated with the progression and increased severity of the disease. Therefore, brain volume reduction in anti-Aβ immunotherapy should be interpreted carefully. As an optional 4th sequence, a 3D T1 is recommended as a baseline MRI and at least once a year during follow-up.
The International Collaboration for Real-World Evidence in AD (ICARE AD)-US study sites for monitoring ARIA suggest that the protocol should include four sequences: 3D T2-FLAIR, 2D T2* GRE, DWI, and 3D T1-weighted anatomic imaging (optional). 3D T1-weighted imaging is recommended to facilitate post-processing and the assessment of disease progression [46].
The scan time in an MRI protocol is also important for surveillance and repeated tests. With the increasing number of patients, the workflow of MRI examinations should be considered. Additionally, long scanning times can reduce patient compliance. Therefore, an appropriate scan time with all the included essential sequences is essential, and we recommend a scan time of 15 minutes.
Differential Diagnosis
CAA-related inflammation and ARIA share a significant overlapping pathophysiology. CAA-related inflammation is an autoimmune encephalopathy secondary to spontaneous autoantibodies targeting Aβ protein deposited in the intracranial vessel wall. It is a potentially reversible condition responsive to corticosteroid therapy [47,48]. The imaging features of CAA-related inflammation are indistinguishable from those of ARIA, including unifocal or multifocal areas of subcortical vasogenic edema with a mild mass effect, except when there is a significant known background of CAA (Fig. 7). Therefore, superimposition on the background of CAA (strictly lobar microhemorrhages, superficial siderosis, and chronic lobar hemorrhage) and a clinical history of its spontaneous occurrence (not drug-induced) are important differential diagnostic points for CAA-related inflammation [49,50].
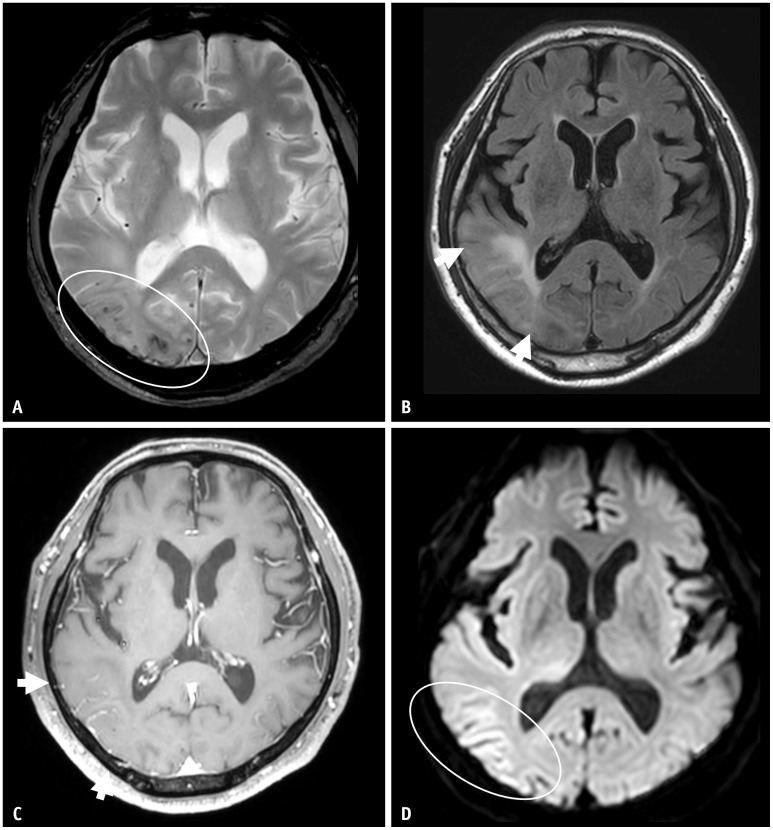
Fig. 7. A 70-year-old male patient presented with confusion. A: On T2* gradient echo sequence, there were multiple lobar distributed cortical and subcortical microbleeds in the right temporooccipital lobes. B: On FLAIR, there was confluent parenchymal high signal intensity (arrows). C: Postcontrast axial T1 images demonstrated marked gyral enhancement in the same area (arrows), suggestive of prominent leptomeningeal vessels. D: On the diffusion-weighted image, there was subtle high signal intensity along the cortex. These images are very similar to amyloid-related imaging abnormalities; however, the patient had not undergone anti-amyloid-beta immunotherapy. The patient was diagnosed with cerebral amyloid angiopathy-related inflammation.
Other conditions in the differential diagnosis of ARIA-E include posterior reversible encephalopathy syndrome (PRES), subacute infarction, and subarachnoid hemorrhage. ARIA-E and PRES have similar imaging characteristics, with a predilection for occipital lobes with petechial hemorrhages; both are reversible. These conditions were differentiated based on clinical history. PRES frequently develops in association with cytotoxic medications, (pre)eclampsia, sepsis, renal disease, and autoimmune disorders. In contrast, ARIA frequently develops in the context of anti-Aβ immunotherapy [16,50].
The parenchymal FLAIR hyperintensity of ARIA-E may be mimicked by ischemia such as subacute infarction. Therefore, DWI is included in the standardized sequence for evaluating ischemic insults. The DWI sequence can easily exclude ischemic conditions accompanied by cytotoxic edema.
The appearance of the sulcal effusion mimicked the imaging features of a subarachnoid hemorrhage. In some early cases, CT and lumbar puncture were performed to detect bleeding. Differentiating ARIA from SAHs requires a systematic clinical approach. Subarachnoid hemorrhage typically presents with severe headache, nausea, and vomiting and may present with a decreased level of consciousness and focal neurological signs. An additional non-contrast CT scan can be used to differentiate acute hemorrhage [50].
The administration of supplemental oxygen and poor CSF signal suppression can mimic ARIA-E. High sulcal signal intensity due to the administration of supplemental oxygen occurs throughout the brain or in a broader region compared to ARIA-E. Poor CSF signal suppression can occur when the patient is not centered in the receiver coil, resulting in artificial hyperintense areas. Therefore, this can be corrected by loading the coil correctly, using a proper size coil for patient size, and shimming to reduce the inhomogeneity of the magnetic field [50].
Management of ARIA
The standard of care is not established for ARIA. Management of ARIA-E generally entails providing symptomatic treatment and close monitoring of the patient’s condition. It is often possible to continue monoclonal antibody therapy for less severe cases while closely monitoring the patients [4]. However, in cases of symptomatic or moderate-to-severe ARIA, therapeutic medication should be temporarily discontinued. Adjunctive therapies, such as corticosteroids, may be employed to mitigate the symptoms. The treatment of CAA-related inflammation, which is hypothesized to share a pathogenic mechanism with ARIA, can be guided by established cases [51]. Although ARIA-H is generally less frequent than ARIA-E, a careful approach is required due to the higher likelihood of severe bleeding events.
Before developing a breakthrough treatment and gaining a comprehensive understanding of the mechanisms underlying ARIA, it is critical to evaluate the risk of ARIA in each patient to make informed treatment decisions. Age, APOE-ε4 allele status, baseline CAA, and anticoagulant use may affect the likelihood of developing ARIA. High-risk individuals should be screened in advance, and patients and caregivers should be thoroughly counseled about their risks. The appropriate recommendation for lecanemab use suggests that patients who cannot undergo MRI due to claustrophobia or metals in the body may not be candidates for lecanemab treatment [11]. It is also crucial to provide patients with information on identifying the possible symptoms of ARIA, including headache, disorientation, and dizziness, to detect and address the condition promptly.
Several studies are underway to reduce the incidence of ARIA. Strategies such as improving drug delivery via brain shuttles and using anti-inflammatory clearance of Aβ by chimeric Gas6 fusion protein to minimize the side effects of an immunologic response are being developed [52,53]. These efforts are expected to provide essential breakthroughs in overcoming ARIA in the future.
CONCLUSION
As therapeutic options for AD progress, approaches to reduce and control ARIA will also advance, ensuring that the advantages of these innovative therapies can be optimized while avoiding potential hazards. To accomplish this, it is critical to increase awareness of ARIA so that patients at high risk of developing ARIA can be screened in advance, monitored during treatment, and promptly treated. In addition, quantitative measurement of ARIA is essential for patient management strategies. However, there is still no tool for the quantitative measurement of ARIA that can be used in a clinical setting. Artificial intelligence-assisted ARIA detection and quantification software has recently been developed and studied. Research on ARIAs using artificial intelligence is promising. Researchers are currently studying biomarkers that have the potential to predict vulnerability to ARIA or to detect its early development. Moreover, improvements in imaging methodologies may offer a more intricate understanding of the formation and evolution of ARIA, thereby facilitating more accurate and proactive treatments.
Footnotes
Conflicts of Interest: Chong Hyun Suh, who holds the respective position as Assistant to the Editor of the Korean Journal of Radiology, was not involved in the editorial evaluation or decision to publish this article. The remaining author has declared no conflicts of interest.
- Conceptualization: So Yeong Jeong, Chong Hyun Suh, Sang Joon Kim, Jae-Sung Lim, Jae-Hong Lee.
- Data curation: So Yeong Jeong, Chong Hyun Suh, Jae-Sung Lim, Jae-Hong Lee.
- Formal analysis: So Yeong Jeong, Chong Hyun Suh, Jae-Sung Lim, Jae-Hong Lee.
- Funding acquisition: Chong Hyun Suh.
- Investigation: So Yeong Jeong, Chong Hyun Suh, Jae-Sung Lim, Jae-Hong Lee.
- Methodology: So Yeong Jeong, Chong Hyun Suh, Jae-Sung Lim, Jae-Hong Lee.
- Project administration: So Yeong Jeong, Chong Hyun Suh, Sang Joon Kim, Jae-Sung Lim, Jae-Hong Lee.
- Resources: So Yeong Jeong, Chong Hyun Suh, Jae-Sung Lim, Jae-Hong Lee.
- Software: So Yeong Jeong, Chong Hyun Suh.
- Supervision: Sang Joon Kim, Cynthia Ann Lemere, Jae-Sung Lim, Jae-Hong Lee.
- Validation: all authors.
- Visualization: So Yeong Jeong, Chong Hyun Suh, Jae-Sung Lim, Jae-Hong Lee.
- Writing—original draft: So Yeong Jeong, Chong Hyun Suh, Jae-Sung Lim, Jae-Hong Lee.
- Writing—review & editing: all authors.
Funding Statement: This work was supported by the National Research Foundation of Korea (NRF-2021R1C1C1014413 to Chong Hyun Suh). This research was supported by a grant of the National Research Foundation of Korea (NRF-2021R1C1C1014413) and a grant of the Korea Dementia Research Project through the Korea Dementia Research Center (KDRC), funded by the Ministry of Health & Welfare and Ministry of Science & ICT, Republic of Korea (RS-2024-00344521).
References
- 1.Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov. 2022;21:306–318. doi: 10.1038/s41573-022-00391-w. [DOI] [PubMed] [Google Scholar]
- 2.Decourt B, Boumelhem F, Pope ED, 3rd, Shi J, Mari Z, Sabbagh MN. Critical appraisal of amyloid lowering agents in AD. Curr Neurol Neurosci Rep. 2021;21:39. doi: 10.1007/s11910-021-01125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong SY, Suh CH, Shim WH, Lim JS, Lee JH, Kim SJ. Incidence of amyloid-related imaging abnormalities in patients with Alzheimer disease treated with anti-β-amyloid immunotherapy: a meta-analysis. Neurology. 2022;99:e2092–e2101. doi: 10.1212/WNL.0000000000201019. [DOI] [PubMed] [Google Scholar]
- 4.Barakos J, Purcell D, Suhy J, Chalkias S, Burkett P, Marsica Grassi C, et al. Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with anti-amyloid beta therapy. J Prev Alzheimers Dis. 2022;9:211–220. doi: 10.14283/jpad.2022.21. [DOI] [PubMed] [Google Scholar]
- 5.Arndt JW, Qian F, Smith BA, Quan C, Kilambi KP, Bush MW, et al. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β. Sci Rep. 2018;8:6412. doi: 10.1038/s41598-018-24501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. Drug approval package: aduhelm (aducanumab-avwa) [accessed on December 30, 2023]. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761178Orig1s000TOC.cfm .
- 8.Logovinsky V, Satlin A, Lai R, Swanson C, Kaplow J, Osswald G, et al. Safety and tolerability of BAN2401--a clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimers Res Ther. 2016;8:14. doi: 10.1186/s13195-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tucker S, Möller C, Tegerstedt K, Lord A, Laudon H, Sjödahl J, et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J Alzheimers Dis. 2015;43:575–588. doi: 10.3233/JAD-140741. [DOI] [PubMed] [Google Scholar]
- 10.van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 11.Cummings J, Apostolova L, Rabinovici GD, Atri A, Aisen P, Greenberg S, et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis. 2023;10:362–377. doi: 10.14283/jpad.2023.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demattos RB, Lu J, Tang Y, Racke MM, Delong CA, Tzaferis JA, et al. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron. 2012;76:908–920. doi: 10.1016/j.neuron.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330:512–527. doi: 10.1001/jama.2023.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperling RA, Jack CR, Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 16.Cogswell PM, Barakos JA, Barkhof F, Benzinger TS, Jack CR, Jr, Poussaint TY, et al. Amyloid-related imaging abnormalities with emerging Alzheimer disease therapeutics: detection and reporting recommendations for clinical practice. AJNR Am J Neuroradiol. 2022;43:E19–E35. doi: 10.3174/ajnr.A7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park HY, Kim KW, Yoon MA, Lee MH, Chae EJ, Lee JH, et al. Role of whole-body MRI for treatment response assessment in multiple myeloma: comparison between clinical response and imaging response. Cancer Imaging. 2020;20:14. doi: 10.1186/s40644-020-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampel H, Elhage A, Cho M, Apostolova LG, Nicoll JAR, Atri A. Amyloid-related imaging abnormalities (ARIA): radiological, biological and clinical characteristics. Brain. 2023;146:4414–4424. doi: 10.1093/brain/awad188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, et al. Consequence of Aβ immunization on the vasculature of human Alzheimer’s disease brain. Brain. 2008;131(Pt 12):3299–3310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- 21.Boncoraglio GB, Piazza F, Savoiardo M, Farina L, DiFrancesco JC, Prioni S, et al. Prodromal Alzheimer’s disease presenting as cerebral amyloid angiopathy-related inflammation with spontaneous amyloid-related imaging abnormalities and high cerebrospinal fluid anti-Aβ autoantibodies. J Alzheimers Dis. 2015;45:363–367. doi: 10.3233/JAD-142376. [DOI] [PubMed] [Google Scholar]
- 22.Piazza F, Greenberg SM, Savoiardo M, Gardinetti M, Chiapparini L, Raicher I, et al. Anti-amyloid β autoantibodies in cerebral amyloid angiopathy-related inflammation: implications for amyloid-modifying therapies. Ann Neurol. 2013;73:449–458. doi: 10.1002/ana.23857. [DOI] [PubMed] [Google Scholar]
- 23.Solopova E, Romero-Fernandez W, Harmsen H, Ventura-Antunes L, Wang E, Shostak A, et al. Fatal iatrogenic cerebral β-amyloid-related arteritis in a woman treated with lecanemab for Alzheimer’s disease. Nat Commun. 2023;14:8220. doi: 10.1038/s41467-023-43933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sotoudeh H, Alizadeh M, Shahidi R, Shobeiri P, Saadatpour Z, Wheeler CA, et al. Imaging spectrum of amyloid-related imaging abnormalities associated with aducanumab immunotherapy. Front Radiol. 2024;3:1305390. doi: 10.3389/fradi.2023.1305390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, et al. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J Alzheimers Dis. 2012;28:49–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- 26.Filippi M, Cecchetti G, Spinelli EG, Vezzulli P, Falini A, Agosta F. Amyloid-related imaging abnormalities and β-amyloid-targeting antibodies: a systematic review. JAMA Neurol. 2022;79:291–304. doi: 10.1001/jamaneurol.2021.5205. [DOI] [PubMed] [Google Scholar]
- 27.Barakos J, Sperling R, Salloway S, Jack C, Gass A, Fiebach JB, et al. MR imaging features of amyloid-related imaging abnormalities. AJNR Am J Neuroradiol. 2013;34:1958–1965. doi: 10.3174/ajnr.A3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brashear HR, Ketter N, Bogert J, Di J, Salloway SP, Sperling R. Clinical evaluation of amyloid-related imaging abnormalities in bapineuzumab phase III studies. J Alzheimers Dis. 2018;66:1409–1424. doi: 10.3233/JAD-180675. [DOI] [PubMed] [Google Scholar]
- 29.Yamada M. Cerebral amyloid angiopathy and gene polymorphisms. J Neurol Sci. 2004;226:41–44. doi: 10.1016/j.jns.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Cummings J, Aisen P, Apostolova LG, Atri A, Salloway S, Weiner M. Aducanumab: appropriate use recommendations. J Prev Alzheimers Dis. 2021;8:398–410. doi: 10.14283/jpad.2021.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salloway S, Marshall GA, Lu M, Brashear HR. Long-term safety and efficacy of bapineuzumab in patients with mild-to-moderate Alzheimer’s disease: a phase 2, open-label extension study. Curr Alzheimer Res. 2018;15:1231–1243. doi: 10.2174/1567205015666180821114813. [DOI] [PubMed] [Google Scholar]
- 32.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barkhof F, Daams M, Scheltens P, Brashear HR, Arrighi HM, Bechten A, et al. An MRI rating scale for amyloid-related imaging abnormalities with edema or effusion. AJNR Am J Neuroradiol. 2013;34:1550–1555. doi: 10.3174/ajnr.A3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bechten A, Wattjes MP, Purcell DD, Aliaga ES, Daams M, Brashear HR, et al. Validation of an MRI rating scale for amyloid-related imaging abnormalities. J Neuroimaging. 2017;27:318–325. doi: 10.1111/jon.12422. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Food and Drug Administration. Prescribing information of ADUHELM. [accessed on January 8, 2024]. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2021/761178s000lbl.pdf .
- 37.Bracoud L, Fiebach JB, Purcell DD, Gass A, Gaensler E, Lindan C, et al. [P1–047]: Validation of a simple severity scale for assessing ARIA-E. [accessed on December 30, 2023]. Available at: [DOI]
- 38.Bracoud L, Klein G, Lyons M, Scelsi MA, Wojtowicz J, Bullain S, et al. Validation of 3- and 5-point severity scales to assess ARIA-E. Alzheimers Dement (Amst) 2023;15:e12503. doi: 10.1002/dad2.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kallmes DF, Hui FK, Mugler JP., 3rd Suppression of cerebrospinal fluid and blood flow artifacts in FLAIR MR imaging with a single-slab three-dimensional pulse sequence: initial experience. Radiology. 2001;221:251–255. doi: 10.1148/radiol.2211001712. [DOI] [PubMed] [Google Scholar]
- 40.Wattjes MP, Lutterbey GG, Harzheim M, Gieseke J, Träber F, Klotz L, et al. Higher sensitivity in the detection of inflammatory brain lesions in patients with clinically isolated syndromes suggestive of multiple sclerosis using high field MRI: an intraindividual comparison of 1.5 T with 3.0 T. Eur Radiol. 2006;16:2067–2073. doi: 10.1007/s00330-006-0195-4. [DOI] [PubMed] [Google Scholar]
- 41.Ayton S. Brain volume loss due to donanemab. Eur J Neurol. 2021;28:e67–e68. doi: 10.1111/ene.15007. [DOI] [PubMed] [Google Scholar]
- 42.Alves F, Kalinowski P, Ayton S. Accelerated brain volume loss caused by anti-β-amyloid drugs: a systematic review and meta-analysis. Neurology. 2023;100:e2114–e2124. doi: 10.1212/WNL.0000000000207156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barkhof F, Knopman DS. Brain shrinkage in anti-β-amyloid Alzheimer trials: neurodegeneration or pseudoatrophy? Neurology. 2023;100:941–942. doi: 10.1212/WNL.0000000000207268. [DOI] [PubMed] [Google Scholar]
- 44.Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66:1356–1360. doi: 10.1212/01.wnl.0000210535.20297.ae. [DOI] [PubMed] [Google Scholar]
- 45.Shoamanesh A, Akoudad S, Himali JJ, Beiser AS, DeCarli C, Seshadri S, et al. Cortical superficial siderosis in the general population: the Framingham heart and Rotterdam studies. Int J Stroke. 2021;16:798–808. doi: 10.1177/1747493020984559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benzinger TL, Barkhof F, Rovira A, Kober T, Whitlow CT, Smith M, et al. Defining a standardized MRI acquisition protocol to be proposed to ICARE AD sites for ARIA monitoring (N3. 001) Neurology. 2022;98(18 supplement):1382 [Google Scholar]
- 47.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat Rev Neurol. 2020;16:30–42. doi: 10.1038/s41582-019-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Auriel E, Charidimou A, Gurol ME, Ni J, Van Etten ES, Martinez-Ramirez S, et al. Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy–related inflammation. JAMA Neurol. 2016;73:197–202. doi: 10.1001/jamaneurol.2015.4078. [DOI] [PubMed] [Google Scholar]
- 49.Wu JJ, Yao M, Ni J. Cerebral amyloid angiopathy-related inflammation: current status and future implications. Chin Med J (Engl) 2021;134:646–654. doi: 10.1097/CM9.0000000000001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal A, Gupta V, Brahmbhatt P, Desai A, Vibhute P, Joseph-Mathurin N, et al. Amyloid-related imaging abnormalities in Alzheimer disease treated with anti-amyloid-β therapy. Radiographics. 2023;43:e230009. doi: 10.1148/rg.230009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Souza A, Tasker K. Inflammatory cerebral amyloid angiopathy: a broad clinical spectrum. J Clin Neurol. 2023;19:230–241. doi: 10.3988/jcn.2022.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung H, Lee SY, Lim S, Choi HR, Choi Y, Kim M, et al. Anti-inflammatory clearance of amyloid-β by a chimeric Gas6 fusion protein. Nat Med. 2022;28:1802–1812. doi: 10.1038/s41591-022-01926-9. [DOI] [PubMed] [Google Scholar]
- 53.Grimm HP, Schumacher V, Schäfer M, Imhof-Jung S, Freskgård PO, Brady K, et al. Delivery of the Brainshuttle™ amyloid-beta antibody fusion trontinemab to non-human primate brain and projected efficacious dose regimens in humans. MAbs. 2023;15:2261509. doi: 10.1080/19420862.2023.2261509. [DOI] [PMC free article] [PubMed] [Google Scholar]