Abstract
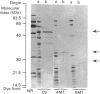
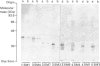
In order to identify the amino acid residues involved in calmodulin (CaM) binding and catalytic activity, rat brain inositol 1,4,5-trisphosphate (InsP3) 3-kinase was expressed in Escherichia coli as a beta-galactosidase fusion protein [clone C5; Takazawa, Vandekerckhove, Dumont & Erneux (1990) Biochem. J. 272, 107-112]. Three deletion mutants in the plasmid of clone C5 were generated using convenient restriction enzymes. The results show that the removal of 34 amino acids from the C-terminal end of InsP3 3-kinase resulted in an inactive protein which still interacted with CaM-Sepharose in a Ca2(+)-dependent way. The catalytic domain is thus located at the C-terminal end of the protein. A series of 5' deletion mutants was prepared and used to produce proteins with the same C-terminal end but shortened N-termini, varying in length by over 80 amino acids. Assay of InsP3 3-kinase activity in bacterial extracts indicated that a maximum of 275 amino acids in the C-terminal region may be sufficient for the construction of a catalytically active domain. Affinity chromatography on CaM-Sepharose of 5' and 3' deletion mutants revealed that the sequence stretching from Ser-156 to Leu-189 is involved in CaM binding and enzyme stimulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi I. C., Kemp B. E., Means A. R. Myosin light chain kinase structure function analysis using bacterial expression. J Biol Chem. 1989 Sep 25;264(27):15843–15849. [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Altin J. G., Karjalainen A., Bygrave F. L. Stimulation of hepatic inositol 1,4,5-trisphosphate kinase activity by Ca2+-dependent and -independent mechanisms. Biochem J. 1988 Dec 15;256(3):697–701. doi: 10.1042/bj2560697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Comte M., Cox J. A., Wollheim C. B. Calcium-calmodulin stimulates inositol 1,4,5-trisphosphate kinase activity from insulin-secreting RINm5F cells. J Biol Chem. 1987 Jul 15;262(20):9437–9440. [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Irvine R. F. Specific binding sites for [3H]inositol(1,3,4,5)tetrakisphosphate on membranes of HL-60 cells. Biochem Biophys Res Commun. 1987 Dec 16;149(2):680–685. doi: 10.1016/0006-291x(87)90421-9. [DOI] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Potter B. V., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 1989 Jul;109(1):85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- Choi K. Y., Kim H. K., Lee S. Y., Moon K. H., Sim S. S., Kim J. W., Chung H. K., Rhee S. G. Molecular cloning and expression of a complementary DNA for inositol 1,4,5-trisphosphate 3-kinase. Science. 1990 Apr 6;248(4951):64–66. doi: 10.1126/science.2157285. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Irvine R. F., Dawson A. P. Synergistic control of Ca2+ mobilization in permeabilized mouse L1210 lymphoma cells by inositol 2,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate. Biochem J. 1990 Oct 15;271(2):549–553. doi: 10.1042/bj2710549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donié F., Reiser G. A novel, specific binding protein assay for quantitation of intracellular inositol 1,3,4,5-tetrakisphosphate (InsP4) using a high-affinity InsP4 receptor from cerebellum. FEBS Lett. 1989 Aug 28;254(1-2):155–158. doi: 10.1016/0014-5793(89)81029-4. [DOI] [PubMed] [Google Scholar]
- Ely J. A., Hunyady L., Baukal A. J., Catt K. J. Inositol 1,3,4,5-tetrakisphosphate stimulates calcium release from bovine adrenal microsomes by a mechanism independent of the inositol 1,4,5-trisphosphate receptor. Biochem J. 1990 Jun 1;268(2):333–338. doi: 10.1042/bj2680333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawler D. J., Potter B. V., Nahorski S. R. Inositol 1,3,4,5-tetrakisphosphate-induced release of intracellular Ca2+ in SH-SY5Y neuroblastoma cells. Biochem J. 1990 Dec 1;272(2):519–524. doi: 10.1042/bj2720519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Elmaoglou-Lazaridou A., Krin E., Ladant D., Bârzu O., Danchin A. Identification of residues essential for catalysis and binding of calmodulin in Bordetella pertussis adenylate cyclase by site-directed mutagenesis. EMBO J. 1989 Mar;8(3):967–972. doi: 10.1002/j.1460-2075.1989.tb03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M., Pollock W. K., Smith P. M., Wreggett K. A. Inositol phosphates: proliferation, metabolism and function. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):281–298. doi: 10.1098/rstb.1988.0077. [DOI] [PubMed] [Google Scholar]
- Johanson R. A., Hansen C. A., Williamson J. R. Purification of D-myo-inositol 1,4,5-trisphosphate 3-kinase from rat brain. J Biol Chem. 1988 Jun 5;263(16):7465–7471. [PubMed] [Google Scholar]
- Joseph S. K., Hansen C. A., Williamson J. R. Inositol 1,3,4,5-tetrakisphosphate increases the duration of the inositol 1,4,5-trisphosphate-mediated Ca2+ transient. FEBS Lett. 1987 Jul 13;219(1):125–129. doi: 10.1016/0014-5793(87)81203-6. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Hansen C. A., Williamson J. R. Inositol tetrakisphosphate mobilizes calcium from cerebellum microsomes. Mol Pharmacol. 1989 Sep;36(3):391–397. [PubMed] [Google Scholar]
- Lee S. Y., Sim S. S., Kim J. W., Moon K. H., Kim J. H., Rhee S. G. Purification and properties of D-myo-inositol 1,4,5-trisphosphate 3-kinase from rat brain. Susceptibility to calpain. J Biol Chem. 1990 Jun 5;265(16):9434–9440. [PubMed] [Google Scholar]
- Li G., Comte M., Wollheim C. B., Cox J. A. Mode of activation of bovine brain inositol 1,4,5-trisphosphate 3-kinase by calmodulin and calcium. Biochem J. 1989 Jun 15;260(3):771–775. doi: 10.1042/bj2600771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. R., Kapiloff M. S., Durgerian S., Tatemoto K., Russo A. F., Hanson P., Schulman H., Rosenfeld M. G. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5962–5966. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 1990 Dec;9(12):3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. J., Downes C. P., Harden T. K., Michell R. H. Turkey erythrocytes possess a membrane-associated inositol 1,4,5-trisphosphate 3-kinase that is activated by Ca2+ in the presence of calmodulin. Biochem J. 1987 Dec 1;248(2):489–493. doi: 10.1042/bj2480489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. How calmodulin binds its targets: sequence independent recognition of amphiphilic alpha-helices. Trends Biochem Sci. 1990 Feb;15(2):59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Ryu S. H., Lee S. Y., Lee K. Y., Rhee S. G. Catalytic properties of inositol trisphosphate kinase: activation by Ca2+ and calmodulin. FASEB J. 1987 Nov;1(5):388–393. doi: 10.1096/fasebj.1.5.2824270. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling T. R. Protein kinases. Regulation by autoinhibitory domains. J Biol Chem. 1990 Feb 5;265(4):1823–1826. [PubMed] [Google Scholar]
- Takazawa K., Lemos M., Delvaux A., Lejeune C., Dumont J. E., Erneux C. Rat brain inositol 1,4,5-trisphosphate 3-kinase. Ca2(+)-sensitivity, purification and antibody production. Biochem J. 1990 May 15;268(1):213–217. doi: 10.1042/bj2680213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa K., Passareiro H., Dumont J. E., Erneux C. Ca2+/calmodulin-sensitive inositol 1,4,5-trisphosphate 3-kinase in rat and bovine brain tissues. Biochem Biophys Res Commun. 1988 Jun 16;153(2):632–641. doi: 10.1016/s0006-291x(88)81142-2. [DOI] [PubMed] [Google Scholar]
- Takazawa K., Passareiro H., Dumont J. E., Erneux C. Purification of bovine brain inositol 1,4,5-trisphosphate 3-kinase. Identification of the enzyme by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Biochem J. 1989 Jul 15;261(2):483–488. doi: 10.1042/bj2610483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa K., Vandekerckhove J., Dumont J. E., Erneux C. Cloning and expression in Escherichia coli of a rat brain cDNA encoding a Ca2+/calmodulin-sensitive inositol 1,4,5-trisphosphate 3-kinase. Biochem J. 1990 Nov 15;272(1):107–112. doi: 10.1042/bj2720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Hirata M., Kuriyama H. Purification and characterization of inositol 1,4,5-trisphosphate 3-kinase from pig aortic smooth muscle. Biochem J. 1988 Apr 1;251(1):129–134. doi: 10.1042/bj2510129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman Y., Howe L. R., Moore J. P., Hesketh T. R., Metcalfe J. C. Calcium regulates inositol 1,3,4,5-tetrakisphosphate production in lysed thymocytes and in intact cells stimulated with concanavalin A. EMBO J. 1987 Apr;6(4):957–962. doi: 10.1002/j.1460-2075.1987.tb04845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]