Abstract
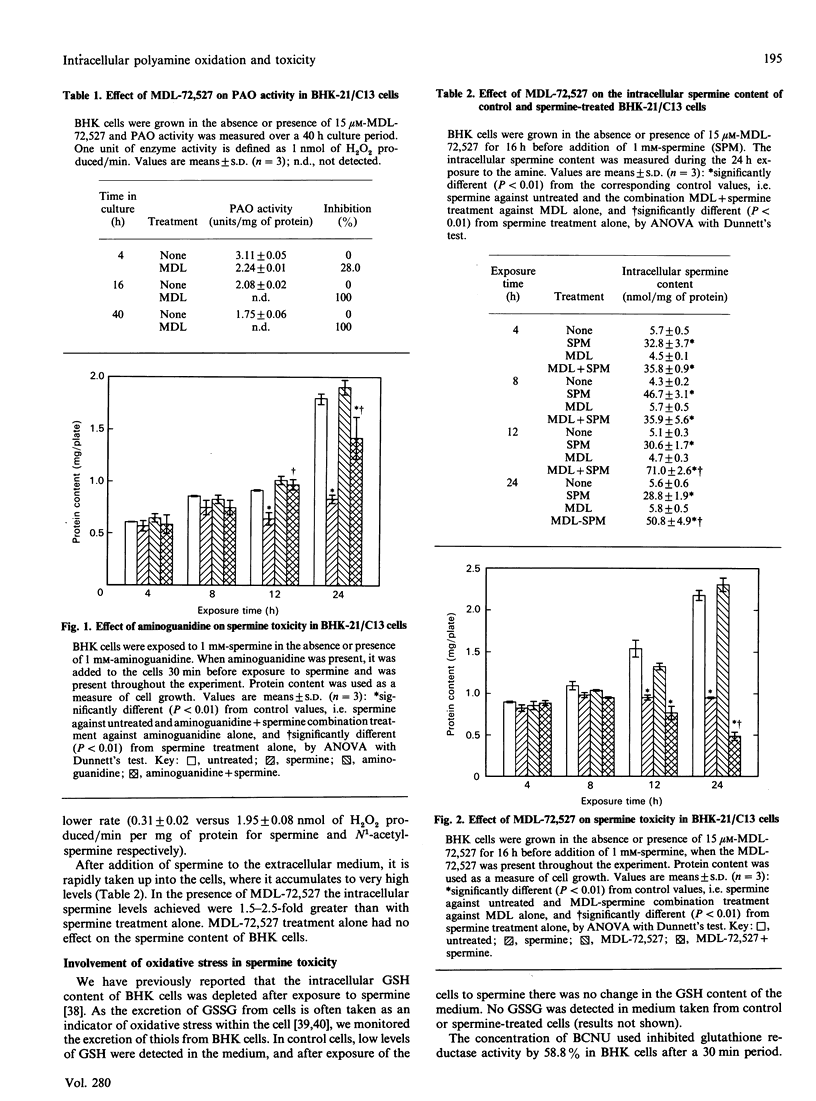
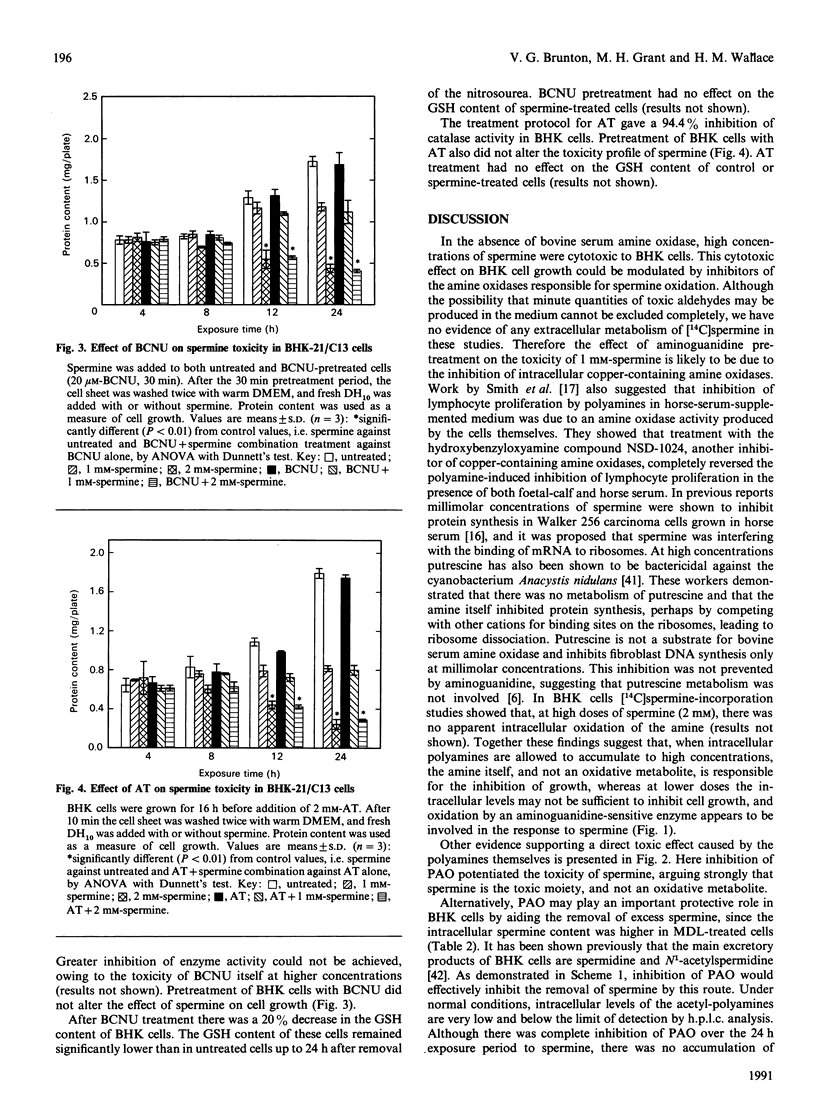
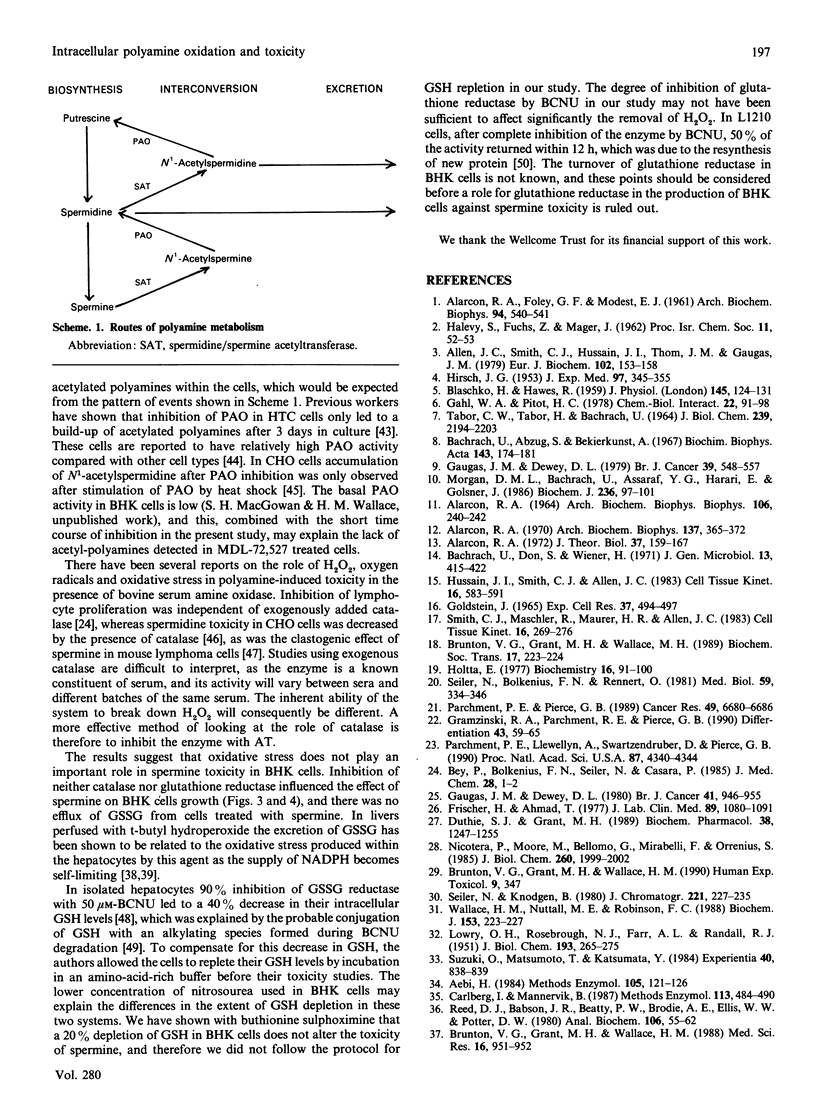
Spermine was toxic to BHK-21/C13 cells in the absence of any extracellular metabolism of the amine. Inhibition of copper-containing amine oxidases with aminoguanidine partially prevented the response, whereas inhibition of polyamine oxidase with MDL-72,527 exacerbated the effect. Oxidation by an intracellular copper-containing amine oxidase may be involved in the toxicity of spermine, whereas the polyamine-interconversion pathway appears to play a cytoprotective role. There was no evidence for spermine imposing a state of oxidative stress within the cells. Inhibition of catalase and glutathione reductase did not alter the cytotoxicity of spermine, and there was no excretion of oxidized glutathione into the extracellular medium. The results suggest that spermine itself can exert a toxic effect directly on the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALARCON R. A., FOLEY G. E., MODEST E. J. Effects of spermine on mammalian cells. Arch Biochem Biophys. 1961 Sep;94:540–541. doi: 10.1016/0003-9861(61)90083-2. [DOI] [PubMed] [Google Scholar]
- ALARCON R. A. ISOLATION OF ACROLEIN FROM INCUBATED MIXTURES OF SPERMINE WITH CALF SERUM AND ITS EFFECTS ON MAMMALIAN CELLS. Arch Biochem Biophys. 1964 Jul 20;106:240–242. doi: 10.1016/0003-9861(64)90183-3. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Alarcon R. A. Acrolein, a component of a universal cell-growth regulatory system: a theory. J Theor Biol. 1972 Oct;37(1):159–167. doi: 10.1016/0022-5193(72)90122-1. [DOI] [PubMed] [Google Scholar]
- Alarcon R. A. Acrolein. IV. Evidence for the formation of the cytotoxic aldehyde acrolein from enzymatically oxidized spermine or spermidine. Arch Biochem Biophys. 1970 Apr;137(2):365–372. doi: 10.1016/0003-9861(70)90450-9. [DOI] [PubMed] [Google Scholar]
- Allen J. C., Smith C. J., Hussain J. I., Thomas J. M., Gaugas J. M. Inhibition of lymphocyte proliferation by polyamines requires ruminant-plasma polyamine oxidase. Eur J Biochem. 1979 Dec;102(1):153–158. doi: 10.1111/j.1432-1033.1979.tb06275.x. [DOI] [PubMed] [Google Scholar]
- BLASCHKO H., HAWES R. Observations on spermine oxidase of mammalian plasma. J Physiol. 1959 Jan 28;145(1):124–131. doi: 10.1113/jphysiol.1959.sp006132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach U., Don S., Wiener H. Antivirus action of acrolein, glutaraldehyde and oxidized spermine. J Gen Virol. 1971 Dec;13(3):415–422. doi: 10.1099/0022-1317-13-3-415. [DOI] [PubMed] [Google Scholar]
- Bey P., Bolkenius F. N., Seiler N., Casara P. N-2,3-Butadienyl-1,4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem. 1985 Jan;28(1):1–2. doi: 10.1021/jm00379a001. [DOI] [PubMed] [Google Scholar]
- Bouzyk E., Rosiek O. Clastogenic and cytotoxic effects of spermine oxidation products in mouse lymphoma L5178Y cells. Cancer Lett. 1988 Feb;39(1):93–99. doi: 10.1016/0304-3835(88)90044-4. [DOI] [PubMed] [Google Scholar]
- Brunton V. G., Grant M. H., Wallace H. M. Spermine toxicity and glutathione depletion in BHK-21/C13 cells. Biochem Pharmacol. 1990 Oct 15;40(8):1893–1900. doi: 10.1016/0006-2952(90)90371-q. [DOI] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Cohen M. B., Duvel D. L. Characterization of the inhibition of glutathione reductase and the recovery of enzyme activity in exponentially growing murine leukemia (L1210) cells treated with 1,3-bis(2-chloroethyl)-1-nitrosourea. Biochem Pharmacol. 1988 Sep 1;37(17):3317–3320. doi: 10.1016/0006-2952(88)90645-4. [DOI] [PubMed] [Google Scholar]
- Duthie S. J., Grant M. H. The toxicity of menadione and mitozantrone in human liver-derived Hep G2 hepatoma cells. Biochem Pharmacol. 1989 Apr 15;38(8):1247–1255. doi: 10.1016/0006-2952(89)90330-4. [DOI] [PubMed] [Google Scholar]
- Eklöw L., Moldéus P., Orrenius S. Oxidation of glutathione during hydroperoxide metabolism. A study using isolated hepatocytes and the glutathione reductase inhibitor 1,3-bis(2-chloroethyl)-1-nitrosourea. Eur J Biochem. 1984 Feb 1;138(3):459–463. doi: 10.1111/j.1432-1033.1984.tb07938.x. [DOI] [PubMed] [Google Scholar]
- Frischer H., Ahmad T. Severe generalized glutathione reductase deficiency after antitumor chemotherapy with BCNU" [1,3-bis(chloroethyl)-1-nitrosourea]. J Lab Clin Med. 1977 May;89(5):1080–1091. [PubMed] [Google Scholar]
- GOLDSTEIN J. THE EFFECT OF SPERMINE ON THE ACCUMULATION OF NUCLEIC ACIDS AND PROTEIN IN MAMMALIAN CELLS. Exp Cell Res. 1965 Feb;37:494–497. doi: 10.1016/0014-4827(65)90199-0. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Pitot H. C. Reversal by aminoguanidine of the inhibition of proliferation of human fibroblasts by spermidine and spermine. Chem Biol Interact. 1978 Jul;22(1):91–98. doi: 10.1016/0009-2797(78)90152-7. [DOI] [PubMed] [Google Scholar]
- Gaugas J. M., Dewey D. L. Evidence for serum binding of oxidized spermine and its potent G1-phase inhibition of cell proliferation. Br J Cancer. 1979 May;39(5):548–557. doi: 10.1038/bjc.1979.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugas J. M., Dewey D. L. Oxygen-dependent free radicals in spermine oxidation cytostatis and chemiluminescence and the role of superoxide dismutase. Br J Cancer. 1980 Jun;41(6):946–955. doi: 10.1038/bjc.1980.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramzinski R. A., Parchment R. E., Pierce G. B. Evidence linking programmed cell death in the blastocyst to polyamine oxidation. Differentiation. 1990 Mar;43(1):59–65. doi: 10.1111/j.1432-0436.1990.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Cohen S. S. Mechanism of toxicity of putrescine in Anacystis nidulans. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3660–3664. doi: 10.1073/pnas.76.8.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Spermine oxidase: an amine oxidase with specificity for spermine and spermidine. J Exp Med. 1953 Mar;97(3):345–355. doi: 10.1084/jem.97.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari P. M., Fuller D. J., Gerner E. W. Heat shock stimulates polyamine oxidation by two distinct mechanisms in mammalian cell cultures. Int J Radiat Oncol Biol Phys. 1989 Feb;16(2):451–457. doi: 10.1016/0360-3016(89)90341-6. [DOI] [PubMed] [Google Scholar]
- Henle K. J., Moss A. J., Nagle W. A. Mechanism of spermidine cytotoxicity at 37 degrees C and 43 degrees C in Chinese hamster ovary cells. Cancer Res. 1986 Jan;46(1):175–182. [PubMed] [Google Scholar]
- Hussain J. I., Smith C. J., Allen J. C. Polyamine-mediated inhibition of in-vitro cell proliferation is not due to acrolein. Cell Tissue Kinet. 1983 Nov;16(6):583–591. [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morgan D. M., Bachrach U., Assaraf Y. G., Harari E., Golenser J. The effect of purified aminoaldehydes produced by polyamine oxidation on the development in vitro of Plasmodium falciparum in normal and glucose-6-phosphate-dehydrogenase-deficient erythrocytes. Biochem J. 1986 May 15;236(1):97–101. doi: 10.1042/bj2360097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Moore M., Bellomo G., Mirabelli F., Orrenius S. Demonstration and partial characterization of glutathione disulfide-stimulated ATPase activity in the plasma membrane fraction from rat hepatocytes. J Biol Chem. 1985 Feb 25;260(4):1999–2002. [PubMed] [Google Scholar]
- Oshino N., Chance B. Properties of glutathione release observed during reduction of organic hydroperoxide, demethylation of aminopyrine and oxidation of some substances in perfused rat liver, and their implications for the physiological function of catalase. Biochem J. 1977 Mar 15;162(3):509–525. doi: 10.1042/bj1620509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchment R. E., Lewellyn A., Swartzendruber D., Pierce G. B. Serum amine oxidase activity contributes to crisis in mouse embryo cell lines. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4340–4344. doi: 10.1073/pnas.87.11.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchment R. E., Pierce G. B. Polyamine oxidation, programmed cell death, and regulation of melanoma in the murine embryonic limb. Cancer Res. 1989 Dec 1;49(23):6680–6686. [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Reed D. J., May H. E., Boose R. B., Gregory K. M., Beilstein M. A. 2-chloroethanol formation as evidence for a 2-chloroethyl alkylating intermediate during chemical degradation of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea and 1-(2-chloroethyl)-3-(trans-4-methylcyclohexyl)-1-nitrosourea. Cancer Res. 1975 Mar;35(3):568–576. [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B., Mamont P. Polyamine oxidase in rat tissues. Biochim Biophys Acta. 1980 Oct;615(2):480–488. doi: 10.1016/0005-2744(80)90514-8. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Rennert O. M. Interconversion, catabolism and elimination of the polyamines. Med Biol. 1981 Dec;59(5-6):334–346. [PubMed] [Google Scholar]
- Seiler N., Knödgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980 Dec 12;221(2):227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- Sies H., Gerstenecker C., Menzel H., Flohé L. Oxidation in the NADP system and release of GSSG from hemoglobin-free perfused rat liver during peroxidatic oxidation of glutathione by hydroperoxides. FEBS Lett. 1972 Oct 15;27(1):171–175. doi: 10.1016/0014-5793(72)80434-4. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Maschler R., Maurer H. R., Allen J. C. Inhibition of cells in culture by polyamines does not depend on the presence of ruminant serum. Cell Tissue Kinet. 1983 May;16(3):269–276. [PubMed] [Google Scholar]
- Suzuki O., Matsumoto T., Katsumata Y. Determination of polyamine oxidase activities in human tissues. Experientia. 1984 Aug 15;40(8):838–839. doi: 10.1007/BF01951981. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., BACHRACH U. IDENTIFICATION OF THE AMINOALDEHYDES PRODUCED BY THE OXIDATION OF SPERMINE AND SPERMIDINE WITH PURIFIED PLASMA AMINE OXIDASE. J Biol Chem. 1964 Jul;239:2194–2203. [PubMed] [Google Scholar]
- Wallace H. M., Macgowan S. H., Keir H. M. Polyamine metabolism in mammalian cells in culture. Biochem Soc Trans. 1985 Apr;13(2):329–330. doi: 10.1042/bst0130329. [DOI] [PubMed] [Google Scholar]
- Wallace H. M., Nuttall M. E., Robinson F. C. Acetylation of spermidine and methylglyoxal bis(guanylhydrazone) in baby-hamster kidney cells (BHK-21/C13). Biochem J. 1988 Jul 1;253(1):223–227. doi: 10.1042/bj2530223. [DOI] [PMC free article] [PubMed] [Google Scholar]


