Abstract
The most frequent mutation of the human hepatitis B virus (HBV) core antigen occurs at amino acid 97. Recently, a phenylalanine (F)-to-leucine (L) mutation at this position (mutant F97L) in HBV surface antigen subtype ayw has been shown to result in an immature secretion phenotype, which is characterized by the nonselective export of an excessive amount of virions containing minus-strand, single-stranded HBV DNA. While subtype ayw mutant F97L has been found in Europe, the major reservoir of HBV resides in Asia and Africa. We report here that the immature secretion phenotype indeed can be found in an HBV strain (subtype adr) prevalent in Asia, changing from an isoleucine (I) to a leucine (mutant I97L). Despite its immature secretion phenotype, the adr variant I97L replicates as well as its parental adr wild-type I97I, supporting the conclusion that the extracellular phenotype of immature secretion is not a consequence of the intracellular HBV DNA replication defect. Further studies demonstrated that it is the acquisition of a leucine, rather than the loss of a wild-type amino acid at codon 97, that is important for immature secretion. We conclude that immature secretion is a subtype-independent phenotype and deficiency in intracellular DNA synthesis is a subtype-dependent phenotype. The former is caused by the trans-acting effect of a mutant core protein, while the latter by a cis-acting effect of a mutated nucleotide on the ayw genome. These immature secretion variants provide an important tool for studying the regulation of HBV virion assembly and secretion.
Human hepatitis B virus (HBV) is the causative agent for viral hepatitis B (25). There are approximately 300 million chronic HBV carriers worldwide. Chronic infection with HBV leads to the development of cirrhosis and liver cancer (27, 28). Genetic variants of HBV have been found frequently in these chronic carriers. Due to some unknown selective pressure, these naturally occurring variants at times exist as a predominant HBV population in chronic carriers. Demonstration of the functional significance of these genetic variants has been difficult since there is no a priori reason to predict what kind of assays available will yield the most informative results. In general, the functional significance of HBV variants has remained largely unknown, despite their strong association with HBV chronicity and pathogenesis.
Naturally occurring mutations of HBV core antigen (HBcAg) gene have been frequently reported (1, 3–6, 8–11, 13, 17, 20, 26, 30, 32–34). Within the core gene, the most frequent mutation occurs at codon 97 (2, 3, 5, 10). To address the functional significance of HBcAg codon 97 variants in patients, we have recently characterized a subtype ayw mutant, F97L, which contains a change from phenylalanine (F) to leucine (L) at amino acid 97 of HBcAg. One characteristic intracellular phenotype of this mutant is the deficiency in plus-strand DNA synthesis resulting in the significant loss in the relaxed circular (RC) DNA form. However, the most surprising is the extracellular phenotype of ayw mutant F97L, which secretes excessive amount of virion particles containing immature HBV genome with a single-stranded (SS) form of DNA (40). In the case of wild-type (wt) hepadnaviruses, it is well known that more mature genomes containing the RC form of DNA are preferentially exported as virion particles (29, 35). For example, when a wt HBV isolate of subtype ayw was assayed in human hepatoma Huh7 cells, we obtained an approximate extracellular RC/SS ratio of 3.82 ± 0.79 (40). In the case of ayw mutant F97L, the extracellular RC/SS ratio is around 0.67 ± 0.20 (i.e., the extracellular SS form becomes more abundant than the RC form in ayw mutant F97L), suggesting that the selectivity for the maturity (or against the immaturity) of the replicating HBV genome during the process of virion secretion may have been altered or lost (40). It should be noted that the immature secretion phenotype, as the term is used here, does not imply the absence of mature genomes in the secreted virions; rather, it indicates an excessive amount of immature genome in the virions relative to the wt HBV. While the mechanism of immature secretion remains to be further investigated, ayw mutant F97L provides a new opportunity to better understand the regulation of HBV virion morphogenesis and secretion. An important question to address is whether the intra- or extracellular phenotype of ayw mutant F97L is caused by the loss of the wt amino acid (F) or by the acquisition of the mutant amino acid (L).
To date, numerous reports have documented the frequent mutation at codon 97 of HBcAg in adr and adw subtypes (1, 3–6, 8–11, 13, 17, 20, 26, 30, 32–34), which changes from an isoleucine (I) to a leucine (L) or phenylalanine (F) (Fig. 1). It remains unclear if the immature secretion phenotype of ayw mutant F97L in Europe (23, 24) can be found universally, such as in the subtype adr or adw mutant 97 prevalent in Asia (1, 3–6, 8–11, 13, 17, 20, 26, 30, 32–34). As shown in Fig. 1, there are at least a total of eight commonly observed amino acid differences in the 183 amino acids of HBcAg between adr and ayw subtypes. Because of the sequence divergence of HBcAg between these different HBV strains, it is difficult to predict if a phenotype observed in one subtype can necessarily be observed in another subtype.
FIG. 1.
Differences in amino acid sequences of HBcAg (or nucleocapsid) between HBV subtypes adr and ayw. The numbers indicate the positions of the diverged amino acids of HBcAg. The most frequent naturally occurring mutation of HBcAg occurs at amino acid 97(∗), changing from isoleucine (I) to leucine (L) in subtype adr and from phenylalanine (F) to leucine in subtype ayw. The sequences of subtypes ayw and adr used in this study are from references 7 and 14, respectively.
In this study, we have attempted to address the aforementioned issues. Our results indicate that in the context of subtype adr, in contrast to subtype ayw, no intracellular viral DNA replication deficiency can be found in adr mutant I97L. Thus, the intracellular viral DNA replication defect of codon 97 mutation appears to be a subtype ayw-dependent phenotype. In contrast, immature secretion appears to be a global and subtype-independent phenotype, resulting solely from the mutant leucine residue at position 97 of HBcAg.
MATERIALS AND METHODS
Plasmid constructs of HBV tandem dimer. (i) pI97I(wt, adr), pI97L (mutant, adr), pI97F (mutant, adr), pF97F(wt, ayw), pF97L(mutant, ayw), and pF97I (mutant, ayw).
Plasmid pI97I, a wt adr HBV tandem dimer construct, is derived from the HBV clone described by Yaginuma et al. (36). Mutagenesis was performed to introduce site-specific mutations by using a commercial kit (Altered Sites II in vitro mutagenesis systems; Promega Co.). The 3.2-kb HBV monomer DNA (adr) was cloned into the BamHI site of pAlter vector, resulting in pAlter-HBVADR, which was subsequently used as a mutagenesis template. The oligonucleotides used to create mutations I97L and I97F (underlined) are 5′-GGG CCT AAA ACT CAG ACA ACT-3′ and 5′-GGG CCT AAA ATT CAG ACA ACT-3′, respectively. Plasmid pF97F, a wt ayw HBV tandem dimer construct, was described as pWT elsewhere (40). The ayw mutant plasmid pF97L was described by Yuan et al. (40). The oligonucleotide used to create mutation F97I is 5′-GGG CCT AAA GAT CAG GCA ACT-3′. Mutant HBV monomers were subsequently dimerized in tandem to mimic the circular configuration of HBV, using a BamHI (for adr) or EcoRI (for ayw) site. All HBV dimer constructs were based on the same vector, pSV2neo. The resulting tandem dimers were confirmed by restriction digestion and DNA sequencing.
(ii) HBV core protein expression vectors.
Plasmids pSVCF97F(wt, ayw), pSVCF97L (mutant, ayw), pSVCF97I (mutant, ayw), pSVCI97I (wt, adr), pSVCI97L (mutant, adr), and pSVCI97F (mutant, adr) are wt and mutant core antigen expression vectors using a simian virus 40 early enhancer and promoter. These vectors were constructed by PCR amplification of the core genes from their corresponding HBV genotypes as described above. The cloning strategy is as described elsewhere (40). Plasmid pSVCF97F was described as pSVC by Yuan et al. (40).
(iii) HBV core protein AUG knockout mutant.
ayw mutant 1903 bears an ablated AUG initiation codon in both copies of the tandem HBV genome and is thus replication defective due to the absence of core protein production (40). Mutant 1903 can be rescued to replicate if core protein is provided by trans complementation.
DNA and RNA probes.
HBV double-strand-, plus-strand-, and minus-strand-specific probes of ayw subtype were prepared as described elsewhere (40). adr-specific probes were prepared as follows.
(i) Double-strand-specific probe.
The full-length 3.1-kb HBV DNA fragment was purified from pAlter-HBV ADR by BamHI digestion. Approximately 25 ng of the 3.1-kb DNA fragment was radiolabeled by using a random-primed DNA labeling kit (Boehringer Mannheim Co.).
(ii) Plus-strand-specific riboprobe.
pAlter-HBV ADR contains a copy of HBV genome of adr subtype. The plus-strand-specific RNA probe was synthesized by using EcoRI-linearized pAlter-HBV ADR DNA and T7 polymerase according to the in vitro transcription procedure recommended by the manufacturer (Amersham Co.).
(iii) Minus-strand-specific riboprobe.
The minus-strand-specific RNA probe was synthesized by using HindIII-linearized pAlter-HBV ADR DNA and SP6 polymerase for in vitro transcription.
Transfection and cell lines.
For DNA transfection, briefly, 10 μg of HBV plasmid DNA was adjusted to a total of 35 μg of DNA with carrier and transfected to human hepatoma cell lines HepG2 and Huh7. Although the experimental results from these two cell lines are qualitatively consistent, we often chose to use HepG2 cell line for the adr experiment simply because we can obtain better adr HBV-specific signal in HepG2 cells than in Huh7 cells. However, in general the degree of HBV genome maturity appears to be higher in adr-HepG2 than in ayw-Huh7 combinations. Preparation of intracellular core particles and viral DNA, and gradient centrifugation analysis of secreted viral particles are detailed elsewhere (38, 39).
Quantitation of the relative abundance of HBV replicative intermediates.
The banding intensity of HBV replicative intermediates in the autoradiogram of Southern blot analysis was measured as described previously (40). Briefly, the images from X-ray film of the RC form population was measured by counting the signal from the 4.0-kb position to a position right above the 1.5-kb position of the SS form, while the intensity of the SS form was measured by counting the signals at and below the 1.5-kb position. The image was acquired from X-ray film using either a Howtek Scanmaster 3+ machine or a Stratagene Eagle Eye II. The stored image was then analyzed with the ONE-DScan computer program (Scanalytics Co., Billerica, Mass.).
RESULTS
The immature secretion phenotype is observed in ayw mutant F97L and adr mutant I97L but not in wt HBV, ayw mutant F97I, and adr mutant I97F.
To see if immature secretion is a truly universal phenomenon among HBV subtypes found in different parts of the world, we have constructed site-directed mutants from wt HBV of both subtypes adr and ayw (Materials and Methods). The media were collected from each transfection of a total of six different naturally occurring genotypes: two wt (adr wt I97I and ayw wt F97F), two adr mutants (I97L and I97F), and two ayw mutants (F97L and F97I). Viral particles in the medium were analyzed by gradient centrifugation and Southern blot analysis (40). As shown in Fig. 2, mature genomes with the RC DNA form are predominant in the virion particle fractions (fractions 10 to 14) in ayw wt F97F and adr wt I97I as well as ayw mutant F97I and adr mutant I97F. The extracellular RC/SS ratio for adr wt in HepG2 cells is about 9.3 ± 2.4, based on four independent transfection experiments (Materials and Methods). This relative abundance between mature RC and immature SS forms in the virion population is consistent with the finding that mature viral genomes are preferentially exported in wt hepadnaviruses (29, 35, 40). In contrast to the wt viruses, ayw mutant F97I, and adr mutant I97F, interestingly, both ayw mutant F97L and adr mutant I97L contain highly abundant immature genomes with the SS form of DNA at equivalent-density fractions. The extracellular RC/SS ratio of adr mutant I97L in HepG2 cells is about 1.5 ± 0.4 (i.e., the RC form is slightly more abundant than the SS form). Therefore, immature genomes with the SS form DNA in adr mutant I97L appear to be enveloped and secreted nonselectively or less selectively than wt (i.e., the export of immature genome in these two mutants is at a surprisingly high efficiency similar to that of the mature genomes with the RC form of DNA). It should also be mentioned here that the HBV DNA signals in fractions 2 to 6 (Fig. 2) represent nonenveloped (or naked) core particles in the medium. Since these naked core particles have never been observed in the sera of patients, the biological significance or relevance of their presence in the tissue culture system remains unclear. In this report, we will focus only on the enveloped virions at fractions 10 to 14. Finally, we noted that ayw mutant F97I secretes a significantly reduced level of virions containing mature genomes (fraction 10 to 14) and does not have the immature secretion phenotype.
FIG. 2.
Immature and mature secretions of Dane particles in HBV variants and wt of different subtypes in gradient centrifugation analysis. Ten micrograms of subtype adr or ayw plasmid DNA was transfected into human hepatoma HepG2 or Huh7cells, respectively. The media were collected on days 5 and 7 posttransfection. Viral particles were then purified from the media through a 20% sucrose cushion and subjected to isopycnic centrifugation in a gradient of 20 to 50% (wt/vol) cesium chloride. Fractions were separated according to their buoyant density and then submitted to HBsAg assay using an AbbottAuszyme EIA kit and HBeAg assay using an Abbott HBe (rDNA) EIA kit (data not shown). The enveloped virions, which are HBsAg positive, band at a density near 1.24 g/cm3 around fractions 10 to 14, while the nonenveloped core particles, which are HBsAg negative and HBcAg positive, band at a density near 1.35 g/cm3 around fractions 2 to 6. The focus of this study is on enveloped virion fractions. Extracellular HBV DNA in every other fraction was detected by a 3.1-kb HBV ayw subtype (upper panel) or adr subtype (lower panel) DNA probe. To reveal the weak HBV DNA signals at fractions 10 and 12 of ayw mutant F97I (upper right panel), prolonged exposure of the X-ray film was necessary, which resulted in the background noise at fractions 16, 18, and 20. RC, full-length relaxed-circle HBV DNA replicative intermediates at 4.0 kb; SS, full-length single-stranded HBV DNA replicative intermediates at 1.5 kb. Based on four independent transfection experiments, the RC/SS ratio of adr mutant I97L in HepG2 cells is close to 1.5 ± 0.4, while the RC/SS ratio of adr wt I97I in HepG2 cells is close to 9.3 ± 2.4. These RC/SS ratios are approximately twofold higher than the previously reported RC/SS ratios of ayw wt F97F and ayw mutant F97L in Huh7 cells (see Discussion).
Quantitative comparisons among these six different genotypes are made possible because of two internal controls: (i) for example, the intracellular HBV DNA replication activities among adr wt I97I and adr variant I97L are more or less equal (Fig. 3, right); (ii) as mentioned above, immature secretion can be defined quantitatively by the RC/SS ratio. Therefore, within each experiment, each value of the RC/SS ratio in different genotypes is internally controlled by itself, and thus the RC/SS value should be independent from experimental variations, such as transfection efficiency.
FIG. 3.
Deficiency of intracellular HBV DNA synthesis is found in the ayw codon 97 mutants but not in the adr counterparts. HBV subtype adr or ayw core particle-associated DNA was harvested 7 days after transfection of human hepatoma Huh7 or HepG2 cells, respectively, and analyzed by Southern blot analysis using a plus-strand-specific riboprobe (upper panel). Following removal of the plus strand-specific riboprobe, the same filter was rehybridized with a minus-strand-specific riboprobe (lower panel). Intracellular core particle-associated HBV DNA was purified with or without micrococcal nuclease or restriction enzyme DpnI treatment (+MN/−DpnI versus −MN/+DpnI). The DpnI treatment can selectively digest the methylated input plasmid DNA originated from bacteria but not the de novo-synthesized HBV DNA originated from transfected hepatoma cells. DpnI-digested plasmid DNA is indicated by ∗.
No defect in DNA replication in adr mutants I97L and I97F.
Previously, we reported pleiotropic effects of mutation F97L in ayw HBV: in addition to the extracellular immature secretion phenotype, we also detected the reduction of the intracellular HBV DNA, particularly the RC form and plus-strand DNA synthesis (40). Since immature secretion was now observed in adr mutant I97L (Fig. 2), we tested whether any similar intracellular deficiency could also be detected in adr mutant I97L by Southern blot analysis. As shown in Fig. 3 (upper and lower right), to our surprise, there was no apparent difference in the intracellular HBV DNA synthesis between adr mutants (I97L and I97F) and the parental wt isolate (I97I). In contrast, both ayw mutants (F97L and F97I) displayed a significant reduction in RC form and plus-strand DNA synthesis (Fig. 3, lower and upper left), and minor reduction in minus-strand DNA synthesis (Fig. 3, lower left), relative to the ayw parental wt isolate (F97F). Previously, some duck hepatitis B virus core antigen mutants exhibited intracellular DNA deficiency, which is actually created by in vitro nuclease digestion during the preparation of viral DNA from physically unstable mutant core particles (15). As shown in Fig. 3 (middle), ayw mutants F97L and F97I exhibited a reduced level of HBV DNA even in the absence of nuclease treatment. Therefore, the intracellular DNA deficiency of ayw mutants F97L and F97I is not primarily caused by the same combined effects of a structurally unstable mutant core particles and the in vitro nuclease digestion.
A cis defect but no trans defect in plus-strand synthesis caused by ayw mutation F97I.
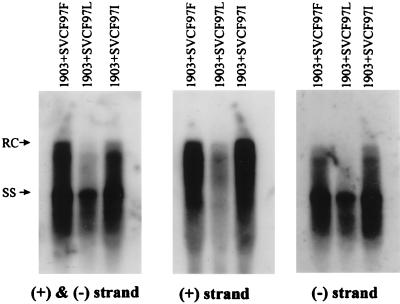
Our recent study of ayw mutant F97L indicates that its intracellular DNA deficiency is caused by both the trans effect of the mutant core protein and the cis effect of a mutant genome (40). Since both ayw mutants F97L and F97I contain a similar intracellular phenotype of RC form and plus-strand deficiency (Fig. 3, upper and lower left), we predicted that the intracellular DNA deficiency of ayw mutant F97I, like that of ayw mutant F97L, is probably caused by both cis and trans effects of the codon 97 mutation. To our surprise, the ayw mutant F97I core protein (SVCF97I), when provided in trans to a core-defective and replication-defective ayw mutant 1903, can rescue the mutant 1903 to a replication level comparable to the rescue by the wt core protein (SVCF97F) (Fig. 4). Therefore, unlike ayw mutant F97L core protein, ayw mutant F97I core protein has no trans effect on HBV RC form and plus-strand DNA synthesis (Fig. 4). Taken together, the results in Fig. 3 and 4 imply only a cis defect in RC form and plus-strand DNA synthesis caused by ayw HBcAg mutation F97I.
FIG. 4.
The deficiency of ayw mutant F97I in intracellular viral DNA synthesis is not caused by a trans defect of its mutant F97I core protein. The ayw mutant F97I core protein was used to trans complement a core gene AUG knockout ayw mutant 1903 (Materials and Methods) via cotransfection into human hepatoma Huh7 cells, and the rescue effect on intracellular HBV DNA synthesis was compared with those for ayw wt or ayw mutant F97L core protein. The ayw wt and ayw mutant F97L and F97I core proteins have been shown to be equally stable in vivo in immunoblot analysis (reference 40 and data not shown). The same filter of intracellular HBV DNA was detected sequentially with three different probes: a minus strand-specific riboprobe, a plus-strand-specific riboprobe, and a 3.1-kb HBV double-strand-specific probe.
Both adr and ayw mutant core proteins containing a leucine residue at amino acid 97 are necessary and sufficient for the immature secretion phenotype.
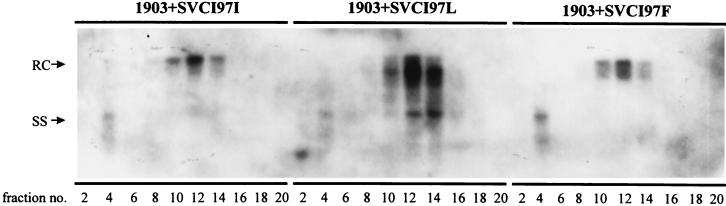
As shown in Fig. 2, we have demonstrated that mutant genotypes F97L (ayw) and I97L (adr), when they exist in an HBV tandem dimer form, exhibit the immature secretion phenotype. In contrast, ayw mutant F97I and adr mutant I97F secrete virions with mature genome in a way qualitatively similar to that of their parental wt viruses. To rigorously prove that the immature secretion is indeed caused by the acquisition of a leucine residue at amino acid 97 of HBcAg in ayw mutant F97L and adr mutant I97L, we needed to dissociate the potential cis effect of a mutant 97 DNA genome (or RNA pregenome) from the potential trans effect of a mutant core protein with a leucine at amino acid 97. To this end, we performed a functional complementation and gradient centrifugation analysis (Fig. 5 and 6). When the core gene AUG knockout mutant 1903 is rescued by cotransfection with different simian virus 40 expression vectors containing various versions of HBV core protein (F97F, F97L, F97I, I97I, I97L, and I97F), only the core protein from ayw mutant F97L (Fig. 5) and adr mutant I97L (Fig. 6) can cause the immature secretion phenotype. In summary, this experiment provides direct evidence that it is the mutant HBcAg with a leucine at amino acid 97, rather than the mutant nucleotide or ribonucleotide at codon 97 of the HBV genome, that is primarily responsible for the immature secretion of enveloped Dane particles.
FIG. 5.
The reduced amount of secreted virions from ayw mutant F97I (containing predominantly mature genomes) is not caused by a trans defect of its mutant core protein. The ayw mutant F97I core protein was used to trans complement ayw core-defective mutant 1903 via cotransfection into human hepatoma Huh7 cells, and the rescue effect on virion secretion was compared with those for wt or ayw mutant F97L core protein. The experimental procedures for the gradient centrifugation analysis were as described for Fig. 2. Part of the naked core particle fractions in this particular experiment rescued by wt core protein (SVCF97F) (upper panel) was accidentally lost during the dialysis procedure.
FIG. 6.
The adr mutant I97L core protein alone is necessary and sufficient to result in the immature secretion of Dane particles. The adr wt I97I and adr mutant I97L and I97F core proteins were supplied in trans to core-defective mutant 1903 via cotransfection into human hepatoma Huh7 cells. The experimental procedures for the gradient centrifugation analysis were as described for Fig. 2.
DISCUSSION
The immature secretion phenotype was first observed in the gradient centrifugation analysis of Dane particles from an ayw HBcAg mutant F97L (40). Here, we demonstrate further that immature secretion is not restricted to the ayw mutant F97L but in fact can be extended to the adr mutant I97L (Fig. 2), suggesting a universal phenomenon among different HBV subtypes.
Subtype-dependent DNA replication deficiency.
In contrast to the aforementioned immature secretion phenotype, the plus-strand deficiency of ayw mutants F97L and F97I is subtype dependent since no similar defect is observed in their counterparts adr mutant I97L and adr wt I97I (Fig. 3; Table 1). HBV genotypes or subtypes have approximately 10% nucleic acid sequence divergence (21). In the case of the ayw and adr plasmids used in this study (7, 14), there are about 300 nucleotide differences between these two different HBV strains (data not shown). Perhaps one general and practical lesson that we have learned here is that the context effect should always be carefully examined in the study of viral variants. When the same mutation at the same position is introduced into two closely related genetic backgrounds, the outcomes in the same functional assay could be surprisingly different. A similar example can be cited in the case of the HBV e-antigen-negative, precore TAG-28 mutation, which occurs more frequently in French patients carrying genotype D than in those carrying genotype A (16). This is probably because the selective pressure against the occurrence of a deleterious TAG-28 mutation within the stem-loop RNA structure of the encapsidation signal is more stringent in genotype A than in genotype D (16, 22, 37).
TABLE 1.
Summary of the subtype-dependent and -independent phenotypes associated with the same mutation at codon 97 of human HBV core gene
| Subtype | Phenotype | Intracellular subtype-dependent plus-strand DNA deficiency? | Extracellular subtype-independent immature secretion? |
|---|---|---|---|
| ayw | F97F (wt) | No | No |
| F97L | Yes | Yes | |
| F97I | Yes | No | |
| adr | I97I (wt) | No | No |
| I97L | No | Yes | |
| I97F | No | No |
Subtype-independent immature secretion.
Among the six different genotypes studied in Fig. 2, 5, and 6, only the ayw mutant F97L and adr mutant I97L displayed similar immature secretion phenotypes, despite their differences in genomic nucleotide sequence context (data not shown). The genome maturation signal hypothesis (29), which was proposed to explain the selective export of the more mature wt duck DHBV genome, is not readily applicable to the core antigen leucine-97 mutants of HBV. In conclusion, as summarized in Table 1, immature secretion is a subtype-independent, HBcAg-dependent, and leucine 97-dependent phenotype.
Replication deficiency of ayw mutant F97I is caused by a cis defect.
Although ayw mutant F97I does not have an immature secretion phenotype (Fig. 2), it does exhibit very weak replication activity (Fig. 3, upper left). However, when the core gene AUG knockout mutant 1903 was rescued by cotransfection with ayw mutant F97I core protein (SVCF97I), the rescued mutant 1903 exhibited both normal level and normal pattern of HBV DNA replicative intermediates, both intracellularly (Fig. 4) and extracellularly (Fig. 5, bottom). Therefore, the barely detectable level of extracellular Dane particles of ayw mutant F97I (Fig. 2, upper right) most likely reflects the significantly reduced intracellular pool size of HBV DNA replicative intermediates (Fig. 3, left). We conclude that the replication defect of ayw mutant F97I is not caused by its mutant core protein and instead is caused by a cis defect of the genome.
Dissociation between the intracellular and extracellular pleiotropic phenotypes of ayw mutant F97L.
Although the intracellular and extracellular levels of HBV DNA could be closely related in the case of ayw mutant F97I, it is unclear if the intracellular profile of plus-strand deficiency of ayw mutant F97L is caused by the highly efficient export (thus leading to depletion) of the intracellular pool of RC form DNA or, conversely, the extracellular Dane particles containing immature genomes are a direct consequence of the intracellular inefficient synthesis of RC form of DNA within the mutant core particles (40) (Fig. 2 and 3). Our unexpected findings that adr mutant I97L has no intracellular DNA synthesis deficiency (Fig. 3) yet maintains the extracellular immature secretion phenotype (Fig. 2 and 5) lend support for an independent, rather than cause-effect, relationship between the intra- and extracellular phenotypes of ayw mutant F97L.
Absolute and relative degree of genome maturity in virions of different HBV subtypes in different cell lines.
The absolute degree of genome maturity of ayw wt virions can be defined by an extracellular RC/SS ratio of around 3.82 ± 0.79, while ayw mutant F97L has an extracellular RC/SS ratio of around 0.67 ± 0.20 in Huh7 cells (40) (Fig. 2). Therefore, one can conclude that the relative degree of genome maturity in ayw mutant F97L is about sixfold lower than that of ayw wt HBV (3.82/0.67). Based on four independent transfection experiments (one of which is shown in Fig. 2), the extracellular RC/SS ratio of adr mutant I97L is 1.5 ± 0.4, while that of adr wt I97I is 9.3 ± 2.4, in HepG2 cells. Therefore, the relative degree of genome maturity in adr mutant I97L virions is also about sixfold lower than that of adr wt I97I (9.3/1.5). Generally speaking, we noted that virion-associated HBV genomes tend to be more mature (higher molecular weight) in HepG2 than in Huh7 cells. In addition, if we compare the middle panels of Fig. 5 and 6, adr-subtype core protein seems to yield more mature virions than ayw subtype protein in the same host cells, at least in the experimental setting of trans complementation. However, in the experimental setting of HBV tandem dimers (Fig. 2), because of the overexposure of this specific X-ray film, the adr-HepG2 combination does not appear to be appreciably more mature than the ayw-Huh7 combination. While the absolute degree of genome maturity of HBV virions depends on multiple parameters, such as the subtypes and cell lines, the relative degrees of genome maturity between adr mutant I97L-HepG2 and ayw mutant F97L-Huh7 are quantitatively similar (both are about sixfold less mature than their parental wt HBV). It should also be mentioned that compared to adr wt I97I and adr mutant I97L, mutant I97F appears to have an intermediate relative degree of genome maturity (about 2.5 fold more mature than adr mutant I97L).
Naked (nonenveloped) core particles.
As shown in Fig. 2, naked core particles at the high-density fractions (fraction 2 to 6) from the CsCl gradient of ayw wt F97F and ayw mutant F97I are more abundant than those of ayw mutant F97L, as measured by the enzyme-linked immunosorbent assay (data not shown). However, in the case of the adr subtype, both wt and mutants I97L and I97F produced naked core particles at a much lower level than the wt ayw. It is possible that these naked core particles are not always physically stable in CsCl gradient centrifugation, and a different gradient system might be better for preserving the integrity of these naked core particles. Since naked core particles have never been observed in the sera of patients, it remains possible that they are released into the media as a result of cell lysis from a small proportion of the transfected cell culture. The biological significance of naked core particles in tissue culture remains to be further investigated.
Biological significance of mutants 97 in patients.
Preferential occurrence of mutations at the so-called domain IV of HBcAg (amino acids 79 to 121) has been found in HBV DNA isolated from liver samples with HBV-related hepatocellular carcinomas (10). In our previous study using a small sampling size (n = 9), approximately 83% of liver samples containing active HBV replication also contain mutations within domain IV. The most frequent missense mutation within domain IV is the substitution at amino acid 97. We noted that this domain IV overlaps with potent T-cell epitopes (12, 18, 19, 31). It should also be mentioned that the emergence of mutant 97 over the wt HBV has been reported in one longitudinal study of a chronic HBV carrier (3). Whether mutant 97 in general is an immune escape variant remains to be tested experimentally. Here, we hypothesize that intracellular DNA deficiency and/or immature secretion of mutant 97 could reduce the total infectious virus titer, leading to the decreased level of HBV-specific target antigens, which consequently could favor the maintenance of chronic infection by lowering the intensity of immune attack.
ACKNOWLEDGMENTS
We thank S. Weaver and colleagues in the Shih laboratory for careful reading of the manuscript. We are indebted to K. Koike for the HBV adr plasmid.
P.-C.T. is supported in part by a J. McLaughlin predoctoral fellowship. We acknoweldge the support of the John Sealy Memorial Endowment Fund. The main part of this work was supported by NIH grant RO1-CA-70336 to C.S.
REFERENCES
- 1.Akarca U S, Lok A S F. Naturally occurring hepatitis B virus core gene mutations. Hepatology. 1995;22:50–60. [PubMed] [Google Scholar]
- 2.Bozkaya H, Ayola B, Lok A S F. High rate of mutations in the hepatitis B core gene during the immune clearance phase of chronic hepatitis B virus infection. Hepatology. 1996;24:32–37. doi: 10.1002/hep.510240107. [DOI] [PubMed] [Google Scholar]
- 3.Chuang W L, Omata M, Ehata T, Yokosuka O, Ito Y, Ohto M. Concentrating missense mutations in core gene of hepatitis B virus. Digest Dis Sci. 1993;38:594–600. doi: 10.1007/BF01316786. [DOI] [PubMed] [Google Scholar]
- 4.Chuang W L, Omata M, Ehata T, Yokosuka O, Ito Y, Imazeki F, Lu S N, Chang W Y, Ohto M. Precore mutations and core clustering mutations in chronic hepatitis B virus infection. Gastroenterology. 1993;104:263–271. doi: 10.1016/0016-5085(93)90861-6. [DOI] [PubMed] [Google Scholar]
- 5.Ehata T, Omata M, Yokosuka O, Hosoda K, Ohto M. Variations in codons 84–101 in the core nucleotide sequence correlate with hepatocellular injury in chronic hepatitis B virus infection. J Clin Investig. 1992;89:332–338. doi: 10.1172/JCI115581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehata T, Omata M, Chuang W L, Yokosuka O, Ito Y, Hosoda K, Ohto M. Mutations in core nucleotide sequence of hepatitis B virus correlate with fulminant and severe hepatitis. J Clin Investig. 1993;91:1206–1213. doi: 10.1172/JCI116281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galibert F, Mandart E, Fitoussi F, Tiollais P, Charnay P. Nucleotide sequence of hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979;281:646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- 8.Gotoh K, Mima S, Uchida T, Shikata T, Yoshizawa K, Irie M, Mizui M. Nucleotide sequence of hepatitis B virus isolated from subjects without serum anti-hepatitis B core antibody. J Med Virol. 1995;46:201–206. doi: 10.1002/jmv.1890460306. [DOI] [PubMed] [Google Scholar]
- 9.Gray A H, Fang J W S, Davis G L, Mizokami M, Wu P C, Williams R, Schuster S M, Lau J Y N. Variations of hepatitis B virus core gene sequence in Western patients with chronic hepatitis B virus infection. J Viral Hepatitis. 1997;4:371–378. doi: 10.1046/j.1365-2893.1997.00075.x. [DOI] [PubMed] [Google Scholar]
- 10.Hosono S, Tai P C, Wang W, Ambrose M, Hwang D, Yuan T T, Peng B H, Yang C S, Lee C S, Shih C. Core antigen mutations of human hepatitis B virus in hepatomas accumulate in MHC class 11-restricted T cell epitopes. Virology. 1995;212:151–162. doi: 10.1006/viro.1995.1463. [DOI] [PubMed] [Google Scholar]
- 11.Hur G M, Lee Y I, Sun D J, Lee J H, Lee Y I. Gradual accumulation of mutations in precore/core region of HBV in patients with chronic active hepatitis: implication of clustering changes in a small region of the HBV core region. J Med Virol. 1996;48:38–46. doi: 10.1002/(SICI)1096-9071(199601)48:1<38::AID-JMV6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Jung M C, Diepolder H M, Spengler U, Wierenga E A, Zachoval R, Zachoval R M, Hoffmann R M, Eichenlaub D, Frosner G, Will H, Pape G R. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J Virol. 1995;69:3358–3368. doi: 10.1128/jvi.69.6.3358-3368.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karasawa T, Shirasawa T, Okawa Y, Kuramoto A, Shimada N, Aizawa Y, Zeniya M, Toda G. Association between frequency of amino acid changes in core region of hepatitis B virus (HBV) and the presence of precore mutation in Japanese HBV carriers. J Gastroenterol. 1997;32:611–622. doi: 10.1007/BF02934110. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Koike K. Complete nucleotide sequence of hepatitis B virus DNA of subtype adr and its conserved gene organization. Gene. 1984;30:227–232. doi: 10.1016/0378-1119(84)90124-0. [DOI] [PubMed] [Google Scholar]
- 15.Kock J, Wieland S, Blum H E, von Weizsacker F. Duck hepatitis B virus nucleocapsids formed by N-terminally extended or C-terminally truncated core proteins disintegrate during viral DNA maturation. J Virol. 1998;72:9116–9120. doi: 10.1128/jvi.72.11.9116-9120.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J S, Tong S P, Wen Y M, Vitvitsk L, Zhang Q, Trepo C. Hepatitis B virus genotype A rarely circulates as an HBe-minus mutant: possible contribution of a single nucleotide in the precore region. J Virol. 1993;67:5402–5410. doi: 10.1128/jvi.67.9.5402-5410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMillan J S, Bowden D S, Angus P W, McCaughan G W, Locarnini S A. Mutations in the hepatitis B virus precore/core and core promoter in patients with severe recurrent disease following liver transplantation. Hepatology. 1996;24:1371–1378. doi: 10.1002/hep.510240610. [DOI] [PubMed] [Google Scholar]
- 18.Menne S, Maschke J, Tolle T K, Lu M, Roggendorf M. Characterization of T-cell response to woodchuck hepatitis core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J Virol. 1997;71:65–74. doi: 10.1128/jvi.71.1.65-74.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milich D R, McLachlan A, Moriarty A, Thornton G B. Immune response to hepatitis B virus core antigen (HBcAg): localization of T cell recognition sites within HBcAg/HBeAg. J Immunol. 1987;139:1223–1231. [PubMed] [Google Scholar]
- 20.Miska S, Gunther S, Vassilev M, Meisel H, Pape G, Will H. Heterogeneity of hepatitis B virus C-gene sequences: Implications for amplification and sequencing. J Hepatol. 1993;18:53–61. doi: 10.1016/s0168-8278(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 21.Ono Y, Onda H, Sasada R, Igarashi K, Sugino Y, Nishioka K. The Complete nucleotide sequences of the cloned hepatitis B virus DNA: subtype adr and adw. Nucleic Acids Res. 1983;11:1747–1757. doi: 10.1093/nar/11.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack J R, Ganem D. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J Virol. 1993;67:3254–3263. doi: 10.1128/jvi.67.6.3254-3263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollicino T, Zanetti A R, Cacciola I, Petit M A, Smedile A, Campo S, Sagliocca L, Pasquali M, Tanzi E, Iongo G, Raimondo G. Pre-S2 defective hepatitis B virus infections in patients with fulminant hepatitis. Hepatology. 1997;26:495–499. doi: 10.1002/hep.510260235. [DOI] [PubMed] [Google Scholar]
- 24.Pollicino T, Campo S, Raimondo G. PreS and core gene heterogeneity in hepatitis B virus (HBV) genomes isolated from patients with long-lasting HBV chronic infection. Virology. 1995;208:672–677. doi: 10.1006/viro.1995.1198. [DOI] [PubMed] [Google Scholar]
- 25.Purcell R H. Hepatitis viruses: changing patterns of human disease. Proc Natl Acad Sci USA. 1994;91:2401–2406. doi: 10.1073/pnas.91.7.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Frias F, Buti M, Jardi R, Cotrina M, Viladomiu L, Esteban R, Guardia J. Hepatitis B virus infection: precore mutants and its relation to viral genotypes and core mutations. Hepatology. 1995;22:1641–1647. doi: 10.1002/hep.1840220605. [DOI] [PubMed] [Google Scholar]
- 27.Shih C, Tai P-C, Whitehead W, Hosono S, Lee C-S, Yang C-S. Hepatitis B and C viruses and liver cancer. In: Bertino J R, editor. Encyclopedia of cancer. II. San Diego, Calif: Academic Press, Inc.; 1997. pp. 824–834. [Google Scholar]
- 28.Slagle B L, Lee T H, Butel J S. Hepatitis B virus and hepatocellular carcinoma. Prog Med Virol. 1992;39:167–203. [PubMed] [Google Scholar]
- 29.Summers J, Mason W S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K, Akahane Y, Hino K, Ohata Y, Mishiro S. Hepatitis B virus genomic sequence in the circulation of hepatocellular carcinoma patients: comparative analysis of 40 full-length isolates. Arch Virol. 1998;143:2313–2326. doi: 10.1007/s007050050463. [DOI] [PubMed] [Google Scholar]
- 31.Tsai S L, Chen M H, Yeh C T, Chu C M, Lin A N, Chiou F H, Chang T H, Liaw Y F. Purification and characterization of a naturally processed hepatitis B virus peptide recognized by CD 8 cytotoxic T lymphocytes. J Clin Investig. 1996;97:577–584. doi: 10.1172/JCI118450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchida T, Aye T T, Becher S O, Hirashima M, Shikata T, Komine F, Moriyama M, Arakawa Y, Takase S, Mima S. Detection of precore/core-mutant hepatitis B virus genome in patients with acute or fulminant hepatitis without serological markers for recent HBV infection. J Hepatol. 1993;18:369–372. doi: 10.1016/s0168-8278(05)80283-1. [DOI] [PubMed] [Google Scholar]
- 33.Uchida T, Aye T T, Shihata T, Yano M, Yatsuhashi H, Koga M, Mima S. Evolution of the hepatitis B virus gene during chronic infection in seven patients. J Med Virol. 1994;43:148–154. doi: 10.1002/jmv.1890430209. [DOI] [PubMed] [Google Scholar]
- 34.Valliammai T, Thyagarajan S P, Zuckerman A J, Harrison T J. Precore and core mutations in HBV from individuals in India with chronic infection. J Med Virol. 1995;45:321–325. doi: 10.1002/jmv.1890450315. [DOI] [PubMed] [Google Scholar]
- 35.Wei Y, Tavis E, Ganem D. Relationship between viral DNA synthesis and virion envelopment in hepatitis B viruses. J Virol. 1996;70:6455–6458. doi: 10.1128/jvi.70.9.6455-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaginuma K, Shirakata Y, Kobayashi M, Koike K. Hepatitis B virus (HBV) particles are produced in a cell culture system by transient expression of transfected HBV DNA. Proc Natl Acad Sci USA. 1987;88:2678–2682. doi: 10.1073/pnas.84.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan T T, Faruqi A, Shih J W K, Shih C. The mechanism of natural occurrence of two closely-linked HBV precore predominant mutations. Virology. 1995;211:144–156. doi: 10.1006/viro.1995.1387. [DOI] [PubMed] [Google Scholar]
- 38.Yuan T-T, Lin M H, Chen D S, Shih C. A defective interference-like phenomenon of human hepatitis B virus in chronic carriers. J Virol. 1998;72:578–584. doi: 10.1128/jvi.72.1.578-584.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan T-T, Lin M H, Qiu S M, Shih C. Functional characterization of naturally occurring variants of human hepatitis B virus containing the core internal deletion mutation. J Virol. 1998;72:2168–2176. doi: 10.1128/jvi.72.3.2168-2176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan T-T, Sahu G K, Whitehead W, Greenberg R, Shih C. The mechanism of an “immature secretion” phenotype of a highly frequent naturally occurring missense mutation at codon 97 of human hepatitis B virus core antigen. J Virol. 1999;73:5731–5740. doi: 10.1128/jvi.73.7.5731-5740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]