Abstract
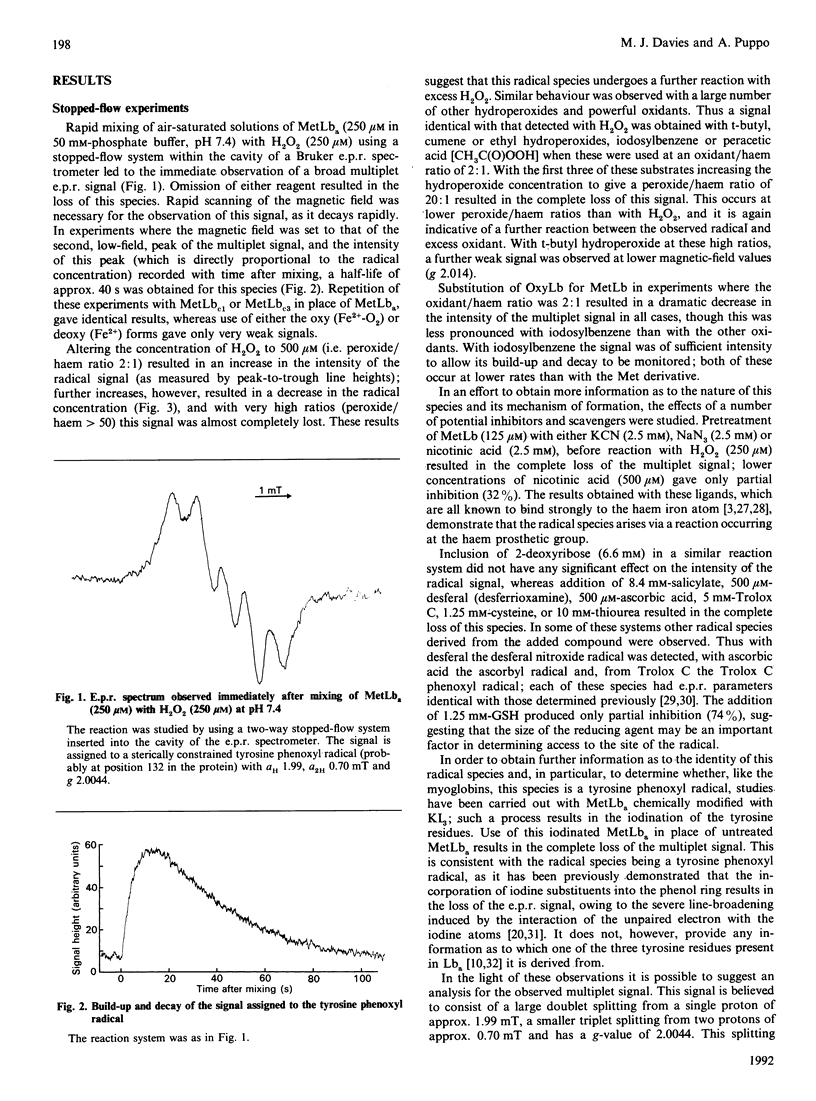
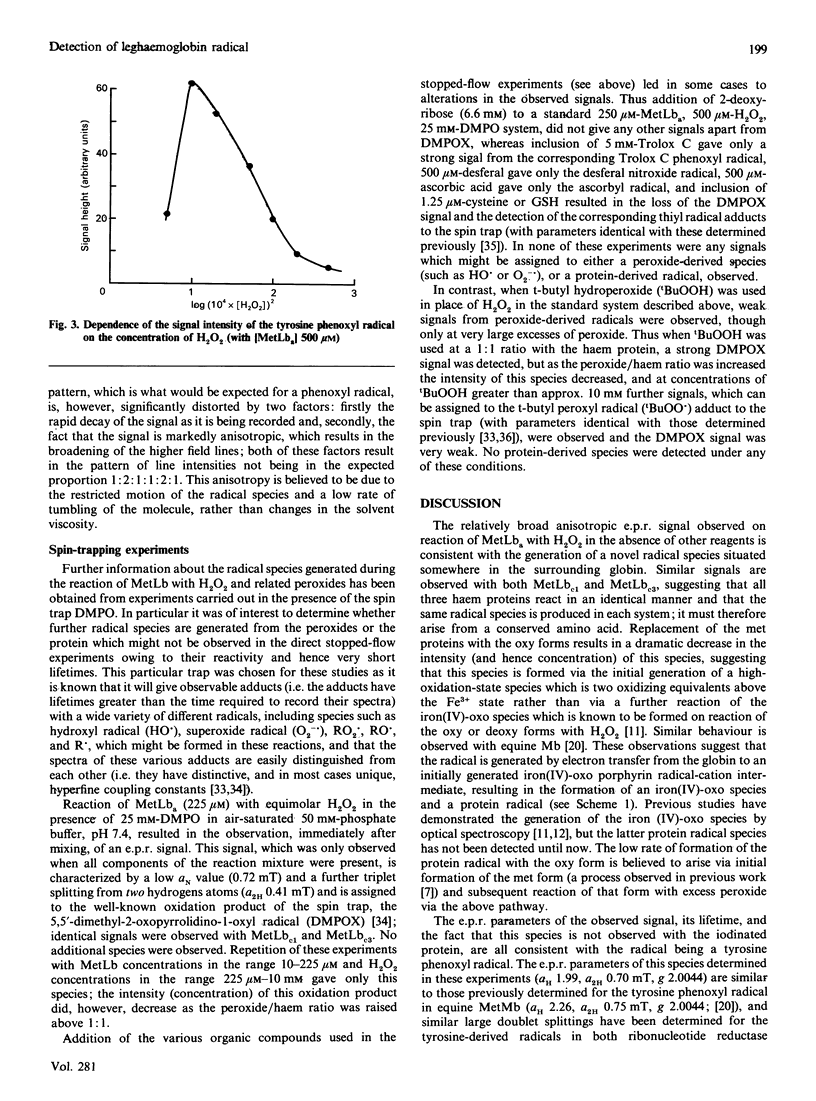
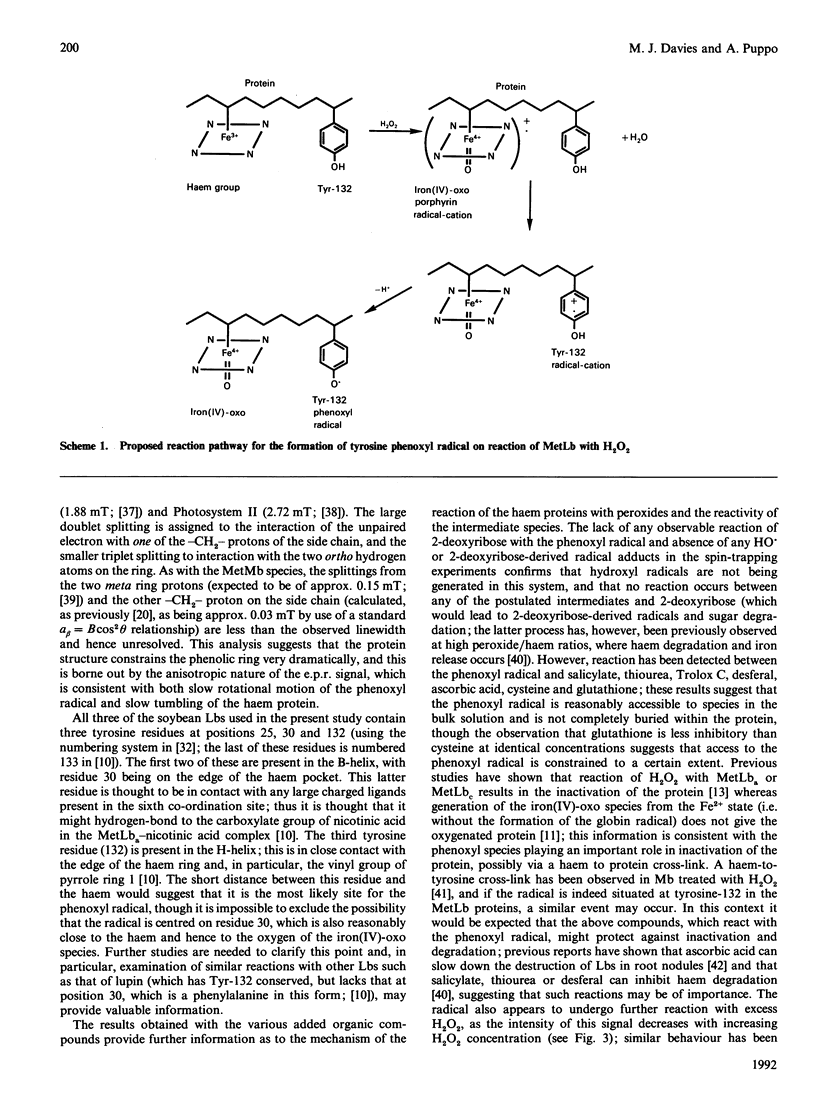
The root nodules of leguminous plants contain an oxygen-carrying protein which is somewhat similar to myoglobin. Reaction of the Fe3+ form of this protein (metleghaemoglobin; MetLb) with H2O2 is known to generate a ferryl [iron(IV)-oxo] species. This intermediate, which is analogous to Compound II of peroxidases and ferryl myoglobin, is one oxidizing equivalent above the initial level. In the present study it is shown that the second oxidizing equivalent from the peroxide is rapidly transferred into the surrounding protein, generating a protein radical which has been detected by e.p.r. spectroscopy; this reaction is analogous to that observed with metmyoglobin. An identical protein-derived species is observed with all three forms of MetLb tested (a, c1, c3) and with a number of other hydroperoxides and two-electron oxidants. This latter result, the observation that the concentration of this species is not affected by certain hydroxyl-radical scavengers, and the loss of the radical when the oxy or deoxy forms are used, demonstrate that this species is formed by electron transfer within the protein rather than by the generation and subsequent reaction of hydroxyl radicals (and related species from the other hydroperoxides). The e.p.r. signal of this species, which decays rapidly with a half-life of approx. 40 s, is consistent with the formation of a sterically constrained tyrosine-derived phenoxyl radical; protein-iodination experiments lend support to this assignment. Reaction between the radical and a number of other compounds has been observed, demonstrating that it is at least partially exposed on the surface of the protein. Analysis of the protein structure suggest that the radical may be centred on a tyrosine residue present at position 132 in the protein; this residue is close to the haem prosthetic group, which would facilitate rapid electron transfer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A., Wittenberg B. A., Wittenberg J. B. Leghemoglobin. II. Changes in conformation and chemical reactivity linked to reaction with a dissociable low molecular weight ligand, X. J Biol Chem. 1973 May 10;248(9):3183–3187. [PubMed] [Google Scholar]
- Aviram I., Wittenberg A., Wittenberg J. B. The reaction of ferrous leghemoglobin with hydrogen peroxide to form leghemoglobin(IV). J Biol Chem. 1978 Aug 25;253(16):5685–5689. [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978 Jul 14;83(1):69–74. doi: 10.1016/0006-291x(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Buettner G. R. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3(4):259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- Catalano C. E., Choe Y. S., Ortiz de Montellano P. R. Reactions of the protein radical in peroxide-treated myoglobin. Formation of a heme-protein cross-link. J Biol Chem. 1989 Jun 25;264(18):10534–10541. [PubMed] [Google Scholar]
- Davies M. J. Detection of myoglobin-derived radicals on reaction of metmyoglobin with hydrogen peroxide and other peroxidic compounds. Free Radic Res Commun. 1990;10(6):361–370. doi: 10.3109/10715769009149905. [DOI] [PubMed] [Google Scholar]
- Davies M. J. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with heme-proteins by electron spin resonance spectroscopy. Biochim Biophys Acta. 1988 Jan 12;964(1):28–35. doi: 10.1016/0304-4165(88)90063-3. [DOI] [PubMed] [Google Scholar]
- Davies M. J. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with rat liver microsomal fractions. Biochem J. 1989 Jan 15;257(2):603–606. doi: 10.1042/bj2570603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J. Direct detection of peroxyl radicals formed in the reactions of metmyoglobin and methaemoglobin with t-butyl hydroperoxide. Free Radic Res Commun. 1989;7(1):27–32. doi: 10.3109/10715768909088158. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Donkor R., Dunster C. A., Gee C. A., Jonas S., Willson R. L. Desferrioxamine (Desferal) and superoxide free radicals. Formation of an enzyme-damaging nitroxide. Biochem J. 1987 Sep 15;246(3):725–729. doi: 10.1042/bj2460725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J., Forni L. G., Shuter S. L. Electron spin resonance and pulse radiolysis studies on the spin trapping of sulphur-centered radicals. Chem Biol Interact. 1987 Feb;61(2):177–188. doi: 10.1016/0009-2797(87)90038-x. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Forni L. G., Willson R. L. Vitamin E analogue Trolox C. E.s.r. and pulse-radiolysis studies of free-radical reactions. Biochem J. 1988 Oct 15;255(2):513–522. [PMC free article] [PubMed] [Google Scholar]
- Davies M. J. Identification of a globin free radical in equine myoglobin treated with peroxides. Biochim Biophys Acta. 1991 Mar 8;1077(1):86–90. doi: 10.1016/0167-4838(91)90529-9. [DOI] [PubMed] [Google Scholar]
- Fuchsman W. H. Discrepancies among published amino acid sequences of soybean leghemoglobins: experimental evidence against cultivar differences as the sources of the discrepancies. Arch Biochem Biophys. 1985 Dec;243(2):454–460. doi: 10.1016/0003-9861(85)90522-3. [DOI] [PubMed] [Google Scholar]
- GEORGE P., IRVINE D. H. The reaction between metmyoglobin and hydrogen peroxide. Biochem J. 1952 Nov;52(3):511–517. doi: 10.1042/bj0520511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K., Yamazaki I. Electron spin resonance spectra of free radicals formed in the reaction of metmyoglobins with ethylhydroperoxide. J Biochem. 1987 Jan;101(1):283–286. doi: 10.1093/oxfordjournals.jbchem.a121903. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P., Ellfolk N. The primary structure of kidney bean leghemoglobin. FEBS Lett. 1974 Jul 15;43(2):239–240. doi: 10.1016/0014-5793(74)81009-4. [DOI] [PubMed] [Google Scholar]
- Nash D. T., Schulman H. M. The absence of oxidized leghemoglobin in soybean root nodules during nodule development. Biochem Biophys Res Commun. 1976 Feb 9;68(3):781–785. doi: 10.1016/0006-291x(76)91213-4. [DOI] [PubMed] [Google Scholar]
- Nishinaga A., Kon H., Cahnmann H. J. Model reactions for the biosynthesis of thyroxine. XI. The nature of a free radical formed in the autoxidation of 4-hydroxy-3,5-diiodophenylpyruvic acid. J Org Chem. 1968 Jan;33(1):157–162. doi: 10.1021/jo01265a029. [DOI] [PubMed] [Google Scholar]
- Puppo A., Halliwell B. Oxidation of dimethylsulphoxide to formaldehyde by oxyhaemoglobin and oxyleghaemoglobin in the presence of hydrogen peroxide is not mediated by "free" hydroxyl radicals. Free Radic Res Commun. 1989;5(4-5):277–281. doi: 10.3109/10715768909074711. [DOI] [PubMed] [Google Scholar]
- Puppo A., Rigaud J., Job D., Ricard J., Zeba B. Peroxidase content of soybean root nodules. Biochim Biophys Acta. 1980 Aug 7;614(2):303–312. doi: 10.1016/0005-2744(80)90220-x. [DOI] [PubMed] [Google Scholar]
- Sievers G., Rönnberg M. Study of the pseudoperoxidatic activity of soybean leghemoglobin and sperm whale myoglobin. Biochim Biophys Acta. 1978 Apr 26;533(2):293–301. doi: 10.1016/0005-2795(78)90376-8. [DOI] [PubMed] [Google Scholar]
- Sjöberg B. M., Reichard P., Gräslund A., Ehrenberg A. The tyrosine free radical in ribonucleotide reductase from Escherichia coli. J Biol Chem. 1978 Oct 10;253(19):6863–6865. [PubMed] [Google Scholar]
- Vainshtein B. K., Harutyunyan E. H., Kuranova I. P., Borisov V. V., Sosfenov N. I., Pavlovsky A. G., Grebenko A. I., Konareva N. V. Structure of leghaemoglobin from lupin root nodules at 5 angstrom resolution. Nature. 1975 Mar 13;254(5496):163–164. doi: 10.1038/254163a0. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Schleyer H. Studies on cytochrome c peroxidase. IX. The reaction of ferrimyoglobin with hydroperoxides and a comparison of peroxide-induced compounds of ferrimyoglobin and cytochrome c peroxidase. J Biol Chem. 1967 Apr 25;242(8):1974–1979. [PubMed] [Google Scholar]


