Abstract
Recombinant adenovirus (Ad) gene transfer vectors are effective at transferring exogenous genes to a variety of cells and tissue types both in vitro and in vivo. However, in the process of gene transfer, the Ad vectors induce the expression of target cell genes, some of which may modify the function of the target cell and/or alter the local milieu. To develop a broader understanding of Ad vector-mediated induction of endogenous gene expression, genes induced by first-generation E1− E4+ Ad vectors in primary human umbilical vein endothelial cells were identified by cDNA subtraction cloning. The identified cDNAs included signaling molecules (lymphoid blast crisis [LBC], guanine nucleotide binding protein α type S [Gα-S], and mitogen kinase [MEK5]), calcium-regulated/cytoskeletal proteins (calpactin p11 and p36 subunits, vinculin, and spinocerebellar ataxia [SCA1]), growth factors (insulin-like growth factor binding protein 4 and transforming growth factor β2), glyceraldehyde-6-phosphate dehydrogenase, an expressed sequence tag, and a novel cDNA showing homology to a LIM domain sequence. Two- to sevenfold induction of the endogenous gene expression was observed at 24 h postinfection, and induction continued up to 72 h, although the timing of gene expression varied among the identified genes. In contrast to that observed in endothelial cells, the Ad vector-mediated induction of gene expression was not found following Ad vector infection of primary human dermal fibroblasts or human alveolar macrophages. Empty Ad capsids did not induce endogenous gene expression in endothelial cells. Interestingly, additional deletion of the E4 gene obviated the upregulation of genes in endothelial cells by the E1− E3− Ad vector, suggesting that genes carried by the E4 region play a central role in modifying target cell gene expression. These findings are consistent with the notion that efficient transfer of exogenous genes to endothelial cells by first-generation Ad vectors comes with the price that these vectors also induce the expression of a variety of cellular genes.
E1− subgroup C adenovirus (Ad) gene transfer vectors are being used extensively to transfer and express genes in a variety of in vitro and in vivo cell targets (1, 15, 74, 78, 81). While these vectors are remarkably efficient at transferring the exogenous gene to the target cell nucleus, and while there is usually robust expression of the transferred gene, the interaction of the vector with the cell may also modify the gene expression of the target cell (12, 51, 55, 61); i.e., while the purpose of using E1− Ad vectors is to modify the genetic repertoire of the target cell, the vector per se may modify the expression of the endogenous genes of the target. In this regard, in vitro studies with a variety of cell lines show that Ad vectors can induce the target cell to express cytokine genes, prolong or reduce cell survival, and activate intracellular signaling pathways (8, 12, 51, 64, 82).
To begin to develop a broader understanding of the interaction of Ad vectors with target cells, we have used cDNA subtraction analysis to evaluate the cellular genes evoked by the interaction of an E1− Ad vector with human umbilical vein endothelial cells (HUVEC). We chose human endothelial cells as the target cells following studies demonstrating that infection of human endothelial cells with E1− E4+ Ad vectors (deleted in E1 sequences but retaining E4 sequences) markedly prolongs the survival of endothelial cells in culture, even in the absence of serum (64). The present study demonstrates that infection of endothelial cells with an E1− E4+ AdNull vector, containing an expression cassette with a cytomegalovirus (CMV) early-intermediate promoter-enhancer but no transgene, induces the expression of a variety of endogenous endothelial cell genes, including those coding for intracellular signaling proteins, calcium-regulated and cytoskeletal proteins, growth-regulating proteins, a housekeeping protein, a known expressed sequenced tag (EST), and a novel cDNA. Interestingly, removal of E4 gene sequences from the Ad vector eliminates the Ad modulation of gene expression in endothelial cells, suggesting that one or more E4 gene products play a role in how the Ad vector modifies the expression of genes endogenous to the target cells.
MATERIALS AND METHODS
Cell culture.
Primary HUVEC were isolated from freshly obtained umbilical cords by collagenase treatment (40) and grown in endothelial cell growth medium (M199 medium containing 20% fetal calf serum, 10 ng of vascular endothelial growth factor [Peprotech, Piscataway, N.J.] per ml, 5 ng of basic fibroblast growth factor [Peprotech] per ml, and 1 U of heparin sulfate [Sigma, St. Louis, Mo.] per ml) at 37°C in a 5% CO2-humidified incubator. Cells from passages 2 to 5 were used in all the experiments. Primary human fibroblasts (HDF-1) were obtained from a skin biopsy specimen from a normal volunteer and cultured in RPMI medium containing 10% fetal calf serum (76). Human alveolar macrophages (AM) were obtained from normal volunteers by bronchoalveolar lavage (68). The lavage fluid was filtered through gauze to remove debris, and the cells were pelleted, washed with phosphate-buffered saline (pH 7.4), and resuspended in RPMI 1640 medium containing 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, and 10 μg of streptomycin per ml. The AM were then purified by adherence to plastic (2 h at 37°C).
Ad vectors.
The Ad vectors used in this study included AdNull (E1− E3− E4+; CMV early-immediate promoter-enhancer, no transgene in the expression cassette) (36), E4− Adβgal (E1−, E3−, E4−; CMV promoter driving the Escherichia coli β-galactosidase (βgal) gene) (7, 82), and E1− E4+ AdGFP (E1− E3− E4+; CMV promoter driving the modified form of the Aeguora victoria green fluorescent protein [GFP]) (28, 86). Ad vector stocks were purified by cesium chloride centrifugation and dialysis and quantified by measurement of PFU in 293 cells as previously described (32, 67). All Ad vectors had a particle/PFU ratio of approximately 100, and all were determined to be free of replication-competent Ad (16). Clinical grade lipopolysaccharide- and endotoxin-free Ad stocks were used for all the experiments. The optimal doses of Ad vector to express genes in >90% of HUVEC, fibroblasts, and macrophages were determined by using AdGFP vector and found to be a multiplicity of infection (MOI) of 50 for HUVEC and fibroblasts and 200 for macrophages. These doses were consistent with those in published reports (37, 42, 64).
Preparation of Ad empty capsids.
To prepare Ad devoid of the Ad genome, crude viral lysate of E1− E4+ AdNull was prepared and purified by cesium chloride centrifugation as described above (32, 67). The top band, containing empty capsids, was collected and purified once more by cesium chloride centrifugation, dialyzed, and stored at −80°C. Spectrophotometric measurements (optical density at 280 nm) were used to calculate the number of empty capsids in the preparation (32). A plaque assay on 293 cells demonstrated the preparation was not contaminated with the starting E1− E4+ AdNull vector.
cDNA subtraction library.
A suppressive subtraction hybridization method was used (PCR-select cDNA subtraction [Clontech, Palo Alto, Calif.]) (20) to isolate cDNAs that are differentially expressed in HUVEC infected with the AdNull vector. Briefly, HUVEC were infected with the AdNull vector at a MOI of 50 or mock infected. After 48 h, poly(A) mRNA was isolated. Poly(A) mRNA (2 μg) was converted to double-stranded cDNA, digested with RsaI, and ligated to adapters. Control (driver) cDNAs and AdNull-infected HUVEC (tester) cDNAs were hybridized at 68°C for 8 h. Fresh driver cDNA was added to the hybridization mixture to enrich differentially expressed sequences. The subtracted cDNA library was constructed by inserting PCR-amplified subtracted cDNA into the T/A cloning vector (Invitrogen, San Diego, Calif.) and differentially screened individually with [32P]dCTP-labeled tester and driver cDNAs. Clones that hybridized strongly with the tester probe but did not hybridize or weakly hybridized to driver probes were isolated and sequenced. BLAST nucleotide homology searches of EST and GenBank nonredundant nucleotide databases were used to help establish the identity of the cDNAs.
Analysis of gene expression.
Gene expression of endothelial cells induced by AdNull infection was analyzed by reverse transcription-PCR (RT-PCR) and Northern analysis. Cultures of endothelial cells were infected with AdNull (MOI of 50) for 90 min at 37°C or exposed to medium that did not contain Ad vector; they were then washed to remove virus, and the culture was continued for 24 to 72 h. As controls, endothelial cells were exposed to Ad empty capsids (5,000 particle units per cell) for 90 min at 37°C and washed, and the incubation was continued for 48 h. For comparison to the endothelial cells, fibroblasts and AM were infected with AdNull (MOI of 50 and 200, respectively) for 90 min at 37°C, the cells were washed, and the incubation was continued for 48 h.
RT-PCRs were used to screen gene expression pattern, and when induction of gene expression was observed, it was confirmed by Northern analysis. For RT-PCR, total RNA (200 ng/reaction) extracted from control or Ad vector-infected cells (48 h postinfection) was reverse transcribed and the resulting cDNA was amplified with gene-specific primers (9600 GeneAmp; Perkin-Elmer). The PCR conditions were 94°C for 30 s (denaturing), 56°C for 1 min (annealing), and 68°C for 2 min (elongation) for 30 cycles. Under these optimal PCR conditions, the internal control glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sense primer, CCTTCATTGACCTCAACTACA; antisense primer, GGCAGTGATGGCATGGCATGGACTGT) amplification was linear and did not reach saturation. DNA contamination was ruled out by pretreatment of the samples with DNase (GIBCO BRL) for 15 min at 37°C and by omitting the reverse transcriptase from the PCR as a control.
For Northern analysis, total RNA was isolated with Trizol reagent (Gibco BRL, Gaithersburg, Md.), and 10 μg of RNA was transferred to Duralon membranes (Stratagene, La Jolla, Calif.) after electrophoresis through a 1% agarose gel under denaturing conditions. Probes were prepared by using gel-purified cDNA fragments isolated from individual clones and labeled with [32P]dCTP by random priming (Stratagene). Hybridizations were performed in Quickhyb solution (Stratagene) for 2 h at 65°C and were followed by sequential washes in 1× SSC (0.15 M sodium chloride, 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate for 30 min and in 0.1×SSC–0.1% sodium dodecyl sulfate for 30 min. Following hybridization, the membranes were analyzed by autoradiography.
RESULTS
Isolation of differentially expressed cDNAs in endothelial cells.
cDNAs of genes that were upregulated in the cells infected with the E1− E4+ AdNull vector were isolated by differential screening of the subtracted library. Of the 300 clones screened, 30 clones were isolated that hybridized strongly to the total cDNA probes derived from the E1− E4+ AdNull vector-infected endothelial cells but did not hybridize or hybridized weakly to total cDNA probes from control uninfected endothelial cells. Of these, 10 cDNAs were identical to previously known sequences, one was identical to a known EST, one was unique (GIA1, for “gene induced by Ad vector”) but showed a high homology to the four-and-a-half-lim-only protein (FHL2) gene, and 16 contained sequences that were derived from the E1− E4+ AdNull vector (Table 1). Of the 12 cDNAs selected for further study, all were represented by a single “hit,” except for lymphoid blast crisis (LBC) cDNA, which was represented by three independent clones containing different regions of the cDNA. The 12 cDNAs corresponding to known sequences were grouped based on their known functions related to intracellular signaling, calcium-regulated cytoskeletal functions, growth regulation, and housekeeping functions.
TABLE 1.
Endothelial-cell cDNAs identified by subtraction analysis and verification by subsequent mRNA analysis of endothelial cells exposed to an E1− E4+ Ad vectora
| Category | Clones | Gene or ESTb | Reference |
|---|---|---|---|
| Intracellular signaling | C8, C9, D8 | LBC | 77 |
| H2 | Gα-S | 35 | |
| H3 | MEK5 | 24 | |
| Ca2+ regulation/cytoskeleton | F8 | Calpactin p11 | 22 |
| E12 | Calpactin p36 | 71 | |
| F7 | Vinculin | 79 | |
| H4 | SCA1 | 3 | |
| Growth regulation | E4 | IGFBP4 | 46 |
| A1 | TGF-β2 | 18 | |
| Housekeeping | G7 | G6PDH | 10 |
| EST | E7 | EST | Accession no. AA557947 |
| Novel cDNAc | F4 | GIA1 | 9 |
Genes identified at 48 h following infection with the AdNull vector (MOI, 50); 16 additional clones contained sequences derived from the E1− E4+ AdNull vector (results not shown).
Where relevant, the specific sequence from the EST database (GenBank) is listed.
GIA1 is a novel cDNA not in the EST and GenBank databases but with homology to four-and-a-half-LIM-only protein (FHL2) (9).
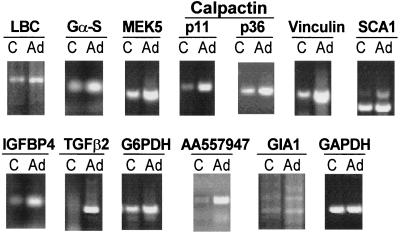
To confirm that the different cDNAs were differentially expressed in endothelial cells following infection with an E1− E4+ Ad vector, levels of the mRNAs representing each gene in uninfected control endothelial cells were compared to those in cells infected with the E1− E4+ AdNull vector. RT-PCR analysis demonstrated that compared to control uninfected cells, the E1− E4+ AdNull vector-infected cells had an induction of expression of LBC, guanine nucleotide binding protein α type S (Gα-S) mitogen kinase 5, (MEK5), calpactin subunits p11 and p36, vinculin, SCA1, insulin-like growth factor binding protein (IGFBP4), and transforming growth factor β2 (TGF-β2), as well as the EST AA557947 and the novel GIA1 gene (Fig. 1). The control GAPDH RNA levels were similar in the control and E1− E4+ Ad vector-infected cells.
FIG. 1.
RT-PCR analysis of endothelial-cell gene expression identified by a subtraction library as being upregulated by an E1− E4+ Ad gene transfer vector. Total RNA (200 ng/reaction) extracted from uninfected control cells (C) or cells infected with E1− E4+ Ad vector (Ad) was used as templates for RT-PCR analysis with gene-specific primers. GAPDH primers were used to confirm RNA integrity and use of equal amounts of RNA. Note that in most cases, there appears to be significant upregulation of expression of the genes identified by the subtraction library.
Effect of E1− E4+ Ad vector infection on gene expression in other primary human cells.
To examine whether the induction of endogenous gene expression by the E1− E4+ Ad vector was a general phenomenon or specific to endothelial cells, primary human skin fibroblasts and alveolar macrophages were infected with the E1− E4+ AdNull vector as for the endothelial cells and relative endogenous gene expression was analyzed by RT-PCR. Infection of >90% of cells was achieved with Ad vector at an MOI of 50 and 200 for fibroblasts and macrophages, respectively. Interestingly, the levels of mRNA corresponding to genes that were observed to be upregulated in the E1− E4+ AdNull vector-infected endothelial cells were not modified in E1− E4+ AdNull vector-infected skin fibroblasts (results not shown), similar to the result observed by Zheng et al. (84) with an E1− E4+ Ad. Likewise, alveolar macrophages infected with the E1− E4+ AdNull vector did not demonstrate the upregulation of genes observed in the endothelial cells (results not shown).
Kinetics of E1− E4+ Ad vector-induced endothelial gene expression.
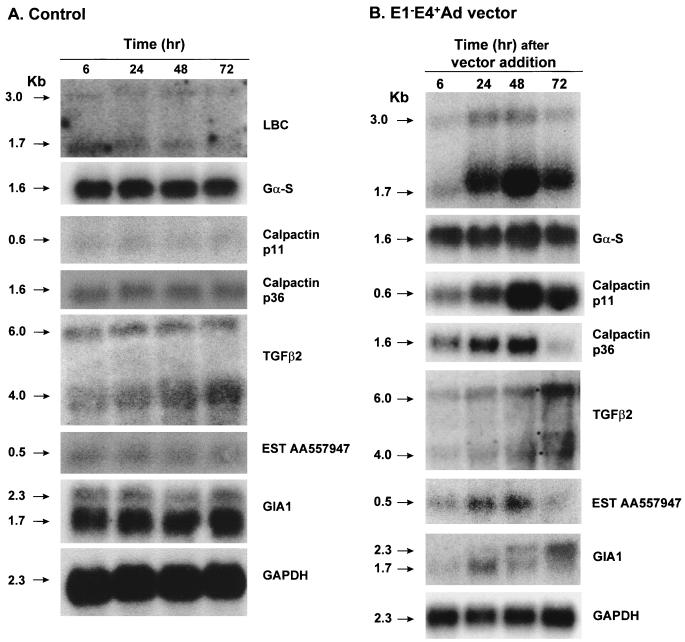
To further evaluate the E1− E4+ Ad vector-induced modification of endogenous endothelial cell gene expression, the kinetics of the genes induced by the vector was evaluated by Northern analysis (Fig. 2). Induction of endogeneous gene expression was first observed 24 h following Ad vector infection. Earlier time points, including 0 h (right after the 90-min infection period [results not shown]) or 6 h postinfection, did not show any induction of gene expression, and the levels were similar to those in uninfected control cells. Several patterns of induction of mRNAs were observed. First, the increased expression of LBC (both 3.0- and 1.7-kb mRNA transcripts) was evident at 24 h postinfection, remained elevated at 48 h, and was declining (but still elevated) by 72 h (Fig. 2). The same pattern was true for the Gα-S, p11, and p36 subunits of calpactin and EST AA557947. Second, a different pattern was observed for TGF-β2, with an increased expression of both the 4.0- and 6.0-kb mRNA transcripts at 24 h that continued to rise over the 72 h of observation. Finally, a third pattern of expression induced by the AdNull vector was that seen for the novel cDNA GIA1, where there was elevation of the small transcript (1.7 kb) at 24 h, which then declined as the levels of a larger transcript (2.3 kb) gradually rose over the 72 h of observation. Gene expression in control cells remained unaltered (Fig. 2).
FIG. 2.
Northern analysis of kinetics of expression of endogenous genes of human endothelial cells following infection by an E1− E4+ Ad vector. (A) Control, uninfected cells. Total RNA (10 μg/lane) was isolated from uninfected control cells over time (6 to 72 h). Each lane was then hybridized to a specific probe as indicated. Note that, other than the 4.0-kb transcript of TGF-β2, there is little change in the transcripts. (B) Cells infected with an E1− E4+ Ad vector. Total RNA was assessed as in panel A. In contrast to panel A, there are marked changes in all transcripts other than GAPDH. The GAPDH probe was used to confirm the integrity and equal loading of RNA.
Endothelial gene expression following addition of empty capsids.
Successful infection of target cells by Ad vectors requires initial interaction of the capsid with the cell surface followed by internalization and transport of the vector DNA to host cell nucleus (33, 42, 49, 74, 80). Since the initial binding of Ad vector capsids to the cell surface is sufficient to activate signal transduction pathways leading to induction of the interleukin-8 gene in HeLa cells (8), we evaluated the effect of empty Ad capsids devoid of vector DNA on endothelial cell gene expression, based on the knowledge that empty capsids (noninfectious Ad particles) bind to target cells in a fashion similar to that of intact, functional Ad (17, 26). Levels of mRNAs corresponding to the subtracted cDNAs were evaluated by RT-PCR in control cells and cells treated with empty capsids 48 h postinfection (data not shown). Compared to control cells, mRNA levels changed very little, with minimal or no increases in mRNA levels of LBC, Gα-S, MEK5, calpactin subunits p11 and p36, vinculin, SCA1, IGFBP4, and TGF-β2. The control GAPDH RNA levels also remained unaltered. Thus, elevated expression of the genes represented by the subtracted cDNAs requires an intact Ad vector, suggesting that Ad components other than just the surface capsid play a role in the observed induction of host cell gene expression.
Requirement of the AdE4 gene for E1− Ad vector induction of endogenous gene expression.
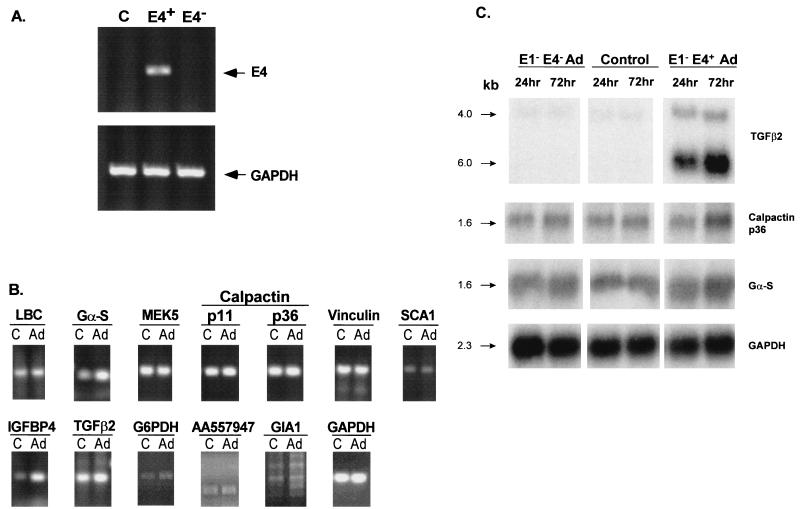
Based on the knowledge that E1− E4+ Ad vectors express low levels of E4 open reading frames (ORF) (2, 6, 30, 43, 60) and that several E4 ORFs have functions that may be linked to host cell transcriptional regulation (21, 34, 38, 74), we hypothesized that E4 region may play a role in the E1− E4+ Ad vector in inducing endogenous endothelial-cell gene expression. To evaluate this hypothesis, induction of endogenous gene expression was analyzed in cells infected with an E1− E4− Ad vector (Fig. 3). RT-PCR analysis of total RNA extracted from cells infected with E1− E4+ Ad vector showed an E4-specific 360-bp PCR product (Fig. 3A). In contrast, neither uninfected control endothelial cells nor endothelial cells infected with E1− E4− Ad vector showed the E4-specific PCR product; i.e., Ad E4 genes are expressed in endothelial cells following infection with an E1− E4+ Ad gene transfer vector but not in the E1− E4− Ad vector-infected or naive control cells.
FIG. 3.
RT-PCR analysis of endogenous gene expression of human endothelial cells following E1− E4− Ad vector infection. (A) Expression of Ad E4 genes in human endothelial cells following infection with an E1− E4− Ad gene transfer vector compared to that with an E1− E4+ vector. Total RNA (200 ng/reaction) extracted from uninfected control cells (C) or cells infected with an E1− E4+ (E4+) or E1− E4− (E4−) Ad gene transfer vectors were used for RT-PCR analysis with AdE4 gene-specific primers. Note that with the E1− E4+ vector but not with the E1− E4− vector, E4 transcripts are observed. (B) Expression of endogenous endothelial genes. The analysis was carried out as in Fig. 1, with total RNA (200 ng/reaction) extracted from uninfected control cells (C) or cells exposed to E1− E4− Ad vector (Ad) used as templates for RT-PCR analysis with gene-specific primers. For both panels A and B, GAPDH primers were used to confirm RNA integrity and use of equal amounts of RNA. (C) Northern analysis of expression of TGF-β2, calpactin p36, and Gα-S expression in endothelial cells infected with E1− E4+ compared to E1− E4− Ad vectors. Total RNA (10 μg) extracted from uninfected control cells or cells infected with either E1− E4+ or E1− E4− Ad gene transfer vectors were used for Northern analysis and probed with TGF-β2, calpactin p36, and Gα-S cDNA probes. Note that there is little change in transcript levels with the E1− E4− vector but there is upregulation with the E1− E4+ vector.
RT-PCR analysis demonstrated that, in contrast to the E1− E4+ Ad vector (Fig. 1), infection of endothelial cells with an E1− E4− Ad vector had little effect on endogenous endothelial gene expression (Fig. 3B). In this regard, while mRNA levels of Gα-S and IGFBP4 were mildly increased in the E1− E4− Ad vector-infected cells, the mRNA levels of the other genes upregulated by the E1− E4+ Ad vector, including the mRNAs for LBC, MEK5, the p11 and p36 subunits of calpactin, vinculin, SCA1, and TGF-β2, remained unaltered (Fig. 3B). Northern blot analysis further confirmed the minimal induction of Gα-S expression in E1− E4− Ad vector-infected cells while calpactin p36 and TGF-β2 expression remained constant (Fig. 3C). Thus, while the modulation of Gα-S gene expression may be independent, to some degree, of AdE4 gene products, the induced expression of other genes corresponding to subtracted cDNAs by E1− E4− Ad vectors appear to require expression of at least some AdE4 gene ORFs.
DISCUSSION
The basic concept underlying the use of any gene transfer strategy is that the vector will deliver the expression cassette with the exogenous gene to the target cell nucleus, where it will be expressed without substantially modifying the integrity and function of the target cell. While it is known that E1− E4+ Ad vectors can achieve delivery and expression of exogenous genes to human endothelium in vitro and in vivo with reasonable efficacy (13, 25, 47, 48, 82), the present study demonstrates that E1− E4+ Ad vectors achieve this at a price, since the vector concomitantly initiates the expression of a number of endogenous endothelial cell genes. By using a subtraction cloning strategy, the data demonstrate that E1− E4+ Ad vectors upregulate a variety of genes that appear to be relatively endothelial-cell specific, since the same vector had little effect on other primary human cells such as fibroblasts and AM. Interestingly, empty Ad capsids did not significantly induce endogenous endothelial-cell gene expression, and comparison of E1− E4+ and E1− E4− Ad vectors demonstrated that the induction of endogenous endothelial genes by the E1− E4+ Ad vector was linked, to a significant degree, to expression of E4 gene products.
Mechanisms of endothelial-cell upregulation of endogenous genes by E1− E4+ Ad vectors.
Ad vector-mediated induction of endothelial-cell gene expression could be mediated either by virus attachment to the cell surface receptors or by Ad viral proteins that are synthesized at very low levels following vector DNA transport to the nucleus. In regard to virus attachment, it has been shown that following addition of an Ad vector to HeLa cells, the Raf/MAP kinase pathway is induced within 20 min of Ad vector binding to the cells; i.e., binding of the Ad vector to the receptor alone is sufficient to activate this pathway (8). However, in regard to endothelial cells, the absence of any significant induction of endothelial gene expression until 24 h following vector infection suggested that the Ad vector binding to its receptor alone was not sufficient, at least for the induction of genes identified by subtraction analysis.
Consistent with this conclusion, empty vector capsids devoid of virus DNA did not induce endothelial-cell gene expression. This observation suggests the requirement of Ad vector DNA to induce the expression of endothelial-cell genes. Because the E1− E4+ but not E1− E4− Ad vector induced endogenous gene expression in endothelial cells, it is likely that AdE4 gene products play a significant role in Ad vector-mediated induction of gene expression. Consistent with this concept, E4 gene expression was detected by RT-PCR in E1− E4+ but not in E1− E4− Ad vector-infected endothelial cells. The molecular mechanisms underlying Ad E4 gene-mediated induction of endogenous gene expression in the endothelial cells are not clear. However, it is known that several proteins encoded by the seven E4 ORFs have functions that regulate cellular transcription. For example, ORF3 protein is associated with the nuclear matrix and is required for accumulation of late viral mRNA in the host cell nucleus (5, 69). ORF6/7 protein modulates the activity of cellular transcription factor E2F (34, 38). ORF4 regulates phosphorylation of the c-fos component of the AP-1 transcription factor (45, 58). Finally, there is recent evidence suggesting that E4 genes may play a role in the persistence of expression of transgenes following in vivo transfer with E1− Ad vectors (6, 30, 43). Thus, although expression of E4 gene is significantly reduced in the absence of E1, the low-level expression of the E4 gene in an E1− E4+ Ad gene transfer vector seem to be sufficient to modulate cellular as well as transgene expression.
Endothelial-cell genes evoked by Ad vectors.
The genes that are upregulated in endothelial cells by an E1− E4+ Ad vector encode proteins that are components of intracellular signaling (LBC, Gα-S, and MEK5), the cytoskeleton (vinculin, calpactin subunits p11 and p36, and SCA1), and growth regulation (IGFBP4 and TGF-β2).
The LBC gene product is known to associate with the GTP binding protein Rho in vivo and functions as a Rho-specific guanine nucleotide exchange factor (84). Consistent with an in vivo role for LBC in Rho regulation, microinjection of the LBC cDNA into quiescent Swiss 3T3 fibroblasts induces actin stress fiber formation indistinguishable from that induced by Rho (84).
The Gα-S subunit of the heterotrimeric G-proteins is a GTPase that mediates the rate-limiting hydrolysis of GTP to GDP (19). Gα-S stimulates adenyl cyclase and increases cellular cyclic AMP levels. Constitutively active mutants of Gα-S-coupled thyrotropin-stimulating hormone receptors have been isolated from hyperfunctioning thyroid adenomas, suggesting a role for Gα-S in the regulation of cell growth (83). Recent evidence also suggests a growth-inhibitory role for constitutively active Gα-S in MCSF-7 breast carcinoma cells (11).
The observation that Gα-S expression in endothelial cells is upregulated by the E1− E4+ Ad vector is consistent with the upregulation of MEK5 expression by the same vector in the same cells. In this context, it is hypothesized that the constitutively active Gα-S might disrupt downstream mitogen-activated protein (MAP) kinase pathways (44, 53). In this pathway, Raf (MAP kinase kinase kinase) phosphorylates and activates MEK (MAP kinase kinase), which in turn mediates the activation of ERK1 and ERK2 (MAP kinase). MEK-5, a novel member of the MEK family, phosphorylates a downstream substrate, big MAP kinase (BMK1), which, in turn, activates myocyte-specific enhancer binding factor (MEF2C), resulting in the induction of the immediate-early response gene c-jun (44).
Vinculin, a 16-kDa single-chain cytoskeletal protein, is organized as linear arrays confined to cell-cell contact areas or as plaques in resting and migrating endothelium (57). Vinculin is a component of a focal adhesion complex which integrates integrin-mediated signaling events (50). Disruption of this complex results in cell death (50). Vinculin is a substrate for several serine/threonine and tyrosine kinases (57) and may play a role in cell growth and transformation. In this context, the exposure of normal rabbit arteries to high concentrations of an E1− E4+ Ad vector upregulates the expression of the adhesion molecules ICAM-1 and VCAM-1 in vascular smooth muscle cells of the arterial wall (61).
Calpactins/lipocortines are a family of calcium binding proteins which interact with phospholipids and cytoskeletal proteins, actin, and spectrins (14). Calpactin I exists as a 36-kDa monomer or as a dimer complexed with an 11-kDa protein (31, 85). The p36 calpactin heavy chain is phosphorylated at a tyrosine residue in transformed and growth factor-stimulated cells (39, 41). Interestingly, both p36 and p11 mRNA levels were upregulated in parallel in the E1− E4+ Ad-infected endothelial cells. Interestingly, there is persistence of parallel upregulation of both subunits in the TGF-β2 upregulates both calpectin p11 and p36 subunits (59).
SCA1 (for “spinocerebellar ataxia 1”), a 200-kDa single-chain protein also referred to as ataxin, is expressed in fetal and adult brain. The neurodegenerative disease spinocerebellar ataxia is associated with expansion of CAG codons in the ataxin coding sequence and consequently a long polyglutamine tract in the ataxin protein. The SCA1 gene is expressed as an 11-kb transcript in all tissues examined (3, 73). We observed that SCA1 is expressed at very low levels in endothelial cells, but the functional role of ataxin in endothelial cells is not known.
Upregulation of IGFBP4 expression by the E1− E4+ Ad vector in primary human endothelial cells suggests a role for this protein in endothelial cell growth and survival. In this regard, IGF-I and IGF-II are structurally related to insulin and mediate the growth promoting-effects of growth hormones, they are essential during fetal development (23, 63). IGF-I is both mitogenic and chemotactic for endothelial cells (4). IGFs are normally bound to high-affinity binding proteins (IGFBP), which inhibit or potentiate the IGF effect depending on the ambient conditions and cell type (63). IGFBP4 is one of the members of this family and is synthesized and secreted by bovine arterial, aortic, and microvessel endothelial cells (56).
The TGF-β family includes multifunctional proteins displaying a variety of activities in a cell-specific manner (54). TGF-β2, a member of the family, is known to be expressed in endothelial cells (72). TGF-β2 is a strong chemoattractant and mitogen for some cell types, but it inhibits endothelial-cell growth and migration (27, 54, 70).
Glyceraldehyde-6-phosphate dehydrogenase (G6PDH) is a rate-limiting enzyme of the hexose monophosphate shunt (66). Expression of G6PDH is under nutritional and hormonal control (29, 52, 62). G6PDH activity is very low under fasting conditions in parenchymal cells, and G6PDH mRNA levels are markedly unregulated following refeeding and insulin administration (52, 62). Hepatic endothelial cells express a very low level of G6PDH under resting conditions and is stimulated severalfold following administration of bacterial endotoxin (75). Interestingly, the present study demonstrates that G6PDH mRNA levels are very low in resting HUVEC but that they are upregulated following infection with an E1− E4+ Ad vector.
Consequences of E1− E4+ Ad vector modification of gene expression.
The specific consequences of the genes observed to be upregulated in endothelial cells by an E1− E4+ Ad vector are unknown. The pattern of E1− E4+ Ad vector induction of genes appears to have at least some cell specificity, in that none of the genes induced by E1− E4+ Ad gene transfer vectors in endothelial cells were upregulated in human primary skin fibroblasts or alveolar macrophages. It is intriguing to speculate on a link between the known function of some of these genes and our recent observation that E1− E4+ Ad vectors suppress the proliferation of primary human endothelial cells in vitro and that primary human endothelial cells infected by E1− E4+ Ad vectors remained viable for prolonged periods even in the absence of serum and growth factors and independent of the transgene carried by the Ad vector (64). Further, E1− E4+ Ad vectors appear to provide an antiapoptotic signal to endothelial cells, in that infection with the vector results in an increase in the ratio of Bcl2 to Bax levels in the endothelial cells. Although the mechanism of these profound effects of E1− E4+ Ad vectors on endothelial-cell survival is unclear, it requires the presence of AdE4 gene products in a fashion similar to that observed in the present study, with the requirement of E4 gene products to induce endothelial-cell genes following infection with the Ad vector.
In view of these observations, at least some of the genes induced by E1− E4+ Ad vectors might play a role in the inhibition of endothelial-cell proliferation and survival following Ad vector infection. For example, the signaling molecules LBC, Gα-S, and MEK5 could initiate a cell survival pathway resulting in prolonged survival of Ad infected endothelial cells. In light of evidence suggesting that cytoskeletal organization and the resulting cell shape play a major role in cell attachment to the substratum and cell survival (65), the cytoskeletal proteins vinculin and calpactins p11 and p36 interact with actin and thus could affect endothelial-cell adhesion and cell shape and hence cell survival. Finally, upregulation of TGF-β2 in Ad-infected endothelial cells might be responsible for the inhibition of cellular DNA synthesis and proliferation.
ACKNOWLEDGMENTS
We thank D. Brough, Gen Vec, Inc., Rockville, Md., for the Ad E1− E4+ vector and N. Mohamed for help in preparing the manuscript.
These studies were supported, in part, by the National Institutes of Health/National Heart, Lung and Blood Institute (grant R01 HL 57318); the Will Rogers Memorial Fund, Los Angeles, Calif.; and Gen Vec, Inc.
REFERENCES
- 1.Anderson W F. Human gene therapy. Nature. 1998;392:25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 2.Armentano D, Zabner J, Sacks C, Sookdeo C C, Smith M P, St. George J A, Wadsworth S C, Smith A E, Gregory R J. Effect of the e4 region on the persistence of transgene expression from adenovirus vectors. J Virol. 1997;71:2408–2416. doi: 10.1128/jvi.71.3.2408-2416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banfi S, Servadio A, Chung M Y, Kwiatkowski T J J, McCall A E, Duvick L A, Shen Y, Roth E J, Orr H T, Zoghbi H Y. Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat Genet. 1994;7:513–520. doi: 10.1038/ng0894-513. [DOI] [PubMed] [Google Scholar]
- 4.Bar R S, Booth B A, Boes M, Dake B L. Insulin-like growth factor-binding proteins from vascular endothelial cells: purification, characterization, and intrinsic biological activities. Endocrinology. 1989;125:1910–1920. doi: 10.1210/endo-125-4-1910. [DOI] [PubMed] [Google Scholar]
- 5.Bridge E, Ketner G. Redundant control of adenovirus late gene expression by early region 4. J Virol. 1989;63:631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brough D E, Hsu C, Kulesa V A, Lee G M, Cantolupo L J, Lizonova A, Kovesdi I. Activation of transgene expression by early region 4 is responsible for a high level of persistent transgene expression from adenovirus vectors in vivo. J Virol. 1997;71:9206–9213. doi: 10.1128/jvi.71.12.9206-9213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brough D E, Lizonova A, Hsu C, Kulesa V A, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol. 1996;70:6497–6501. doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruder J T, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan K K, Tsui S K, Lee S M, Luk S C, Liew C C, Fung K P, Waye M M, Lee C Y. Molecular cloning and characterization of FHL2, a novel LIM domain protein preferentially expressed in human heart. Gene. 1998;210:345–350. doi: 10.1016/s0378-1119(97)00644-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen E Y, Cheng A, Lee A, Kuang W J, Hillier L, Green P, Schlessinger D, Ciccodicola A, D’Urso M. Sequence of human glucose-6-phosphate dehydrogenase cloned in plasmids and a yeast artificial chromosome. Genomics. 1991;10:792–800. doi: 10.1016/0888-7543(91)90465-q. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Bander J A, Santore T A, Chen Y, Ram P T, Smit M J, Iyengar R. Expression of Q227L-galphas in MCF-7 human breast cancer cells inhibits tumorigenesis. Proc Natl Acad Sci USA. 1998;95:2648–2652. doi: 10.1073/pnas.95.5.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clesham G J, Adam P J, Proudfoot D, Flynn P D, Efstathiou S, Weissberg P L. High adenoviral loads stimulate NF kappaB-dependent gene expression in human vascular smooth muscle cells. Gene Ther. 1998;5:174–180. doi: 10.1038/sj.gt.3300576. [DOI] [PubMed] [Google Scholar]
- 13.Crawford L E, Milliken E E, Irani K, Zweier J L, Becker L C, Johnson T M, Eissa N T, Crystal R G, Finkel T, Goldschmidt-Clermont P J. Superoxide-mediated actin response in post-hypoxic endothelial cells. J Biol Chem. 1996;271:26863–26867. doi: 10.1074/jbc.271.43.26863. [DOI] [PubMed] [Google Scholar]
- 14.Crompton M R, Moss S E, Crumpton M J. Diversity in the lipocortin/calpactin family. Cell. 1988;55:1–3. doi: 10.1016/0092-8674(88)90002-5. [DOI] [PubMed] [Google Scholar]
- 15.Crystal R G. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 16.Crystal R G, McElvaney N G, Rosenfeld M A, Chu C S, Mastrangeli A, Hay J G, Brody S L, Jaffe H A, Eissa N T, Danel C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994;8:42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- 17.Defer C, Belin M T, Caillet-Boudin M L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Martin R, Haendler B, Hofer-Warbinek R, Gaugitsch H, Wrann M, Schlusener H, Seifert J M, Bodmer S, Fontana A, Hofer E. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J. 1987;6:3673–3677. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhanasekaran N, Tsim S T, Dermott J M, Onesime D. Regulation of cell proliferation by G proteins. Oncogene. 1998;17:1383–1394. doi: 10.1038/sj.onc.1202242. [DOI] [PubMed] [Google Scholar]
- 20.Diatchenko L, Lau Y F, Campbell A P, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov E D, Siebert P D. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 22.Dooley T P, Weiland K L, Simon M. cDNA sequence of human p11 calpactin I light chain. Genomics. 1992;13:866–868. doi: 10.1016/0888-7543(92)90171-n. [DOI] [PubMed] [Google Scholar]
- 23.Elgin R G, Busby W H J, Clemmons D R. An insulin-like growth factor (IGF) binding protein enhances the biologic response to IGF-I. Proc Natl Acad Sci USA. 1987;84:3254–3258. doi: 10.1073/pnas.84.10.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.English J M, Vanderbilt C A, Xu S, Marcus S, Cobb M H. Isolation of MEK5 and differential expression of alternatively spliced forms. J Biol Chem. 1995;270:28897–28902. doi: 10.1074/jbc.270.48.28897. [DOI] [PubMed] [Google Scholar]
- 25.Erzurum S C, Lemarchand P, Rosenfeld M A, Yoo J H, Crystal R G. Protection of human endothelial cells from oxidant injury by adenovirus-mediated transfer of the human catalase cDNA. Nucleic Acids Res. 1993;21:1607–1612. doi: 10.1093/nar/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fender P, Ruigrok R W H, Gout E, Buffet S, Chroboczek J. Adenovirus dodecahedron, a new vector for human gene transfer. Nat Biotechnol. 1999;15:52–56. doi: 10.1038/nbt0197-52. [DOI] [PubMed] [Google Scholar]
- 27.Frater-Schroder M, Muller G, Birchmeier W, Bohlen P. Transforming growth factor-beta inhibits endothelial cell proliferation. Biochem Biophys Res Commun. 1986;137:295–302. doi: 10.1016/0006-291x(86)91209-x. [DOI] [PubMed] [Google Scholar]
- 28.Frey B M, Rafii S, Teterson M, Eaton D, Crystal R G, Moore M A S. Adenovector-mediated expression of human thrombopoietin cDNA in immune-compromised mice: insights into the pathophysiology of osteomyelofibrosis. J Immunol. 1998;160:691–699. [PubMed] [Google Scholar]
- 29.Fritz R S, Stumpo D J, Kletzien R F. Glucose-6-phosphate dehydrogenase mRNA sequence abundance in primary cultures of rat hepatocytes. Effect of insulin and dexamethasone. Biochem J. 1986;237:617–619. doi: 10.1042/bj2370617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao G P, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glenney J R J. Calpactins: calcium-regulated membrane-skeletal proteins. Bioessays. 1987;7:173–175. doi: 10.1002/bies.950070408. [DOI] [PubMed] [Google Scholar]
- 32.Graham F L, Prevec L. Manipulation of adenovirus vectors. In: Murray E J, editor. Methods in molecular biology. Clifton, N.J: The Humana Press; 1991. pp. 109–128. [DOI] [PubMed] [Google Scholar]
- 33.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 34.Hardy S, Engel D A, Shenk T. An adenovirus early region 4 gene product is required for induction of the infection-specific form of cellular E2F activity. Genes Dev. 1989;3:1062–1074. doi: 10.1101/gad.3.7.1062. [DOI] [PubMed] [Google Scholar]
- 35.Harris B A. Complete cDNA sequence of a human stimulatory GTP-binding protein alpha subunit. Nucleic Acids Res. 1988;16:3585. doi: 10.1093/nar/16.8.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hersh J, Crystal R G, Bewig B. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther. 1995;2:124–131. [PubMed] [Google Scholar]
- 37.Hidaka C, Milano E, Leopold P L, Bergelson J M, Hackett N R, Finberg R W, Wickham T J, Kovesdi I, Roelvink P, Crystal R G. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J Clin Investig. 1999;103:579–587. doi: 10.1172/JCI5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang M M, Hearing P. The adenovirus early region 4 open reading frame 6/7 protein regulates the DNA binding activity of the cellular transcription factor, E2F, through a direct complex. Genes Dev. 1989;3:1699–1710. doi: 10.1101/gad.3.11.1699. [DOI] [PubMed] [Google Scholar]
- 39.Isacke C M, Trowbridge I S, Hunter T. Modulation of p36 phosphorylation in human cells: studies using anti-p36 monoclonal antibodies. Mol Cell Biol. 1986;6:2745–2751. doi: 10.1128/mcb.6.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Investig. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnsson N, Nguyen V P, Soling H D, Weber K. Functionally distinct serine phosphorylation sites of p36, the cellular substrate of retroviral protein kinase: differential inhibition of reassociation with p11. EMBO J. 1986;5:3455–3460. doi: 10.1002/j.1460-2075.1986.tb04669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaner R J, Worgall S, Leopold P L, Stolze E, Milano E, Hidaka C, Ramalingam R, Hackett N R, Singh R, Bergelson J, Finberg R, Falck-Pedersen E, Crystal R G. Modification of the genetic program of human alveolar macrophages by adenovirus vectors in vitro is feasible but inefficient, limited in part by the low level of expression of the Coxsackie/adenovirus receptor. Am J Respir Cell Mol Biol. 1999;20:361–370. doi: 10.1165/ajrcmb.20.3.3398. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan J M, Armentano D, Sparer T E, Wynn S G, Peterson P A, Wadsworth S C, Couture K K, Pennington S E, St. George J A, Gooding L R, Smith A E. Characterization of factors involved in modulating persistence of transgene expression from recombinant adenovirus in the mouse lung. Hum Gene Ther. 1997;8:45–56. doi: 10.1089/hum.1997.8.1-45. [DOI] [PubMed] [Google Scholar]
- 44.Kato Y, Kravchenko V V, Tapping R I, Han J, Ulevitch R J, Lee J D. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleinberger T, Shenk T. Adenovirus E4orf4 protein binds to protein phosphatase 2A, and the complex down regulates E1A-enhanced junB transcription. J Virol. 1993;67:7556–7560. doi: 10.1128/jvi.67.12.7556-7560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.La Tour D, Mohan S, Linkhart T A, Baylink D J, Strong D D. Inhibitory insulin-like growth factor-binding protein: cloning, complete sequence, and physiological regulation. Mol Endocrinol. 1990;4:1806–1814. doi: 10.1210/mend-4-12-1806. [DOI] [PubMed] [Google Scholar]
- 47.Lemarchand P, Jaffe H A, Danel C, Cid M C, Kleinman H K, Stratford-Perricaudet L D, Perricaudet M, Pavirani A, Lecocq J P, Crystal R G. Adenovirus-mediated transfer of a recombinant human alpha 1-antitrypsin cDNA to human endothelial cells. Proc Natl Acad Sci USA. 1992;89:6482–6486. doi: 10.1073/pnas.89.14.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemarchand P, Jones M, Danel C, Yamada I, Mastrangeli A, Crystal R G. In vivo adenovirus-mediated gene transfer to lungs via pulmonary artery. J Appl Physiol. 1994;76:2840–2845. doi: 10.1152/jappl.1994.76.6.2840. [DOI] [PubMed] [Google Scholar]
- 49.Leopold P L, Ferris B, Grinberg I, Worgall S, Hackett N R, Crystal R G. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum Gene Ther. 1998;9:367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- 50.Levkau B, Herren B, Koyama H, Ross R, Raines E W. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J Exp Med. 1998;187:579–586. doi: 10.1084/jem.187.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lieber A, He C Y, Meuse L, Schowalter D, Kirillova I, Winther B, Kay M A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manos P, Nakayama R, Holten D. Regulation of glucose-6-phosphate dehydrogenase synthesis and mRNA abundance in cultured rat hepatocytes. Biochem J. 1991;276:245–250. doi: 10.1042/bj2760245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 54.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 55.McElvaney N G, Crystal R G. IL-6 release and airway administration of human CFTR cDNA adenovirus vector. Nat Med. 1995;1:182–184. doi: 10.1038/nm0395-182b. [DOI] [PubMed] [Google Scholar]
- 56.Moser D R, Lowe W L J, Dake B L, Booth B A, Boes M, Clemmons D R, Bar R S. Endothelial cells express insulin-like growth factor-binding proteins 2 to 6. Mol Endocrinol. 1992;6:1805–1814. doi: 10.1210/mend.6.11.1282670. [DOI] [PubMed] [Google Scholar]
- 57.Muhs A, Noll T, Piper H M. Vinculin phosphorylation and barrier failure of coronary endothelial monolayers under energy depletion. Am J Physiol. 1997;273:H608–H617. doi: 10.1152/ajpheart.1997.273.2.H608. [DOI] [PubMed] [Google Scholar]
- 58.Muller U, Kleinberger T, Shenk T. Adenovirus E4orf4 protein reduces phosphorylation of c-Fos and E1A proteins while simultaneously reducing the level of AP-1. J Virol. 1992;66:5867–5878. doi: 10.1128/jvi.66.10.5867-5878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munz B, Gerke V, Gillitzer R, Werner S. Differential expression of the calpactin I subunits annexin II and p11 in cultured keratinocytes and during wound repair. J Invest Dermatol. 1997;108:307–312. doi: 10.1111/1523-1747.ep12286470. [DOI] [PubMed] [Google Scholar]
- 60.Nevins J R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981;26:213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- 61.Newman K D, Dunn P F, Owens J W, Schulick A H, Virmani R, Sukhova G, Libby P, Dichek D A. Adenovirus-mediated gene transfer into normal rabbit arteries results in prolonged vascular cell activation, inflammation, and neointimal hyperplasia. J Clin Investig. 1995;96:2955–2965. doi: 10.1172/JCI118367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prostko C R, Fritz R S, Kletzien R F. Nutritional regulation of hepatic glucose-6-phosphate dehydrogenase. Transient activation of transcription. Biochem J. 1989;258:295–299. doi: 10.1042/bj2580295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajaram S, Baylink D J, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 64.Ramalingam R, Rafii S, Worgall S, Rough D E, Crystal R G. E1− E4+ adenoviral gene transfer vectors function as a “pro-life” signal to promote survival of primary human endothelial cells. Blood. 1999;93:2936–2944. [PubMed] [Google Scholar]
- 65.Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, Colotta F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J Cell Biol. 1994;127:537–546. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rognstad R, Katz J. Effects of 2,4-dihydroxybutyrate on lipogenesis in rat hepatocytes. J Biol Chem. 1979;254:11969–11972. [PubMed] [Google Scholar]
- 67.Rosenfeld M A, Yoshimura K, Trapnell B C, Yoneyama K, Rosenthal E R, Dalemans W, Fukayama M, Bargon J, Stier L E, Stratford-Perricaudet L, Perricaudet M, Guggino W B, Pavirani A, Lecocq J-P, Crystal R G. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 68.Russi T J, Crystal R G. Pulmonary macrophages. In: Crystal R G, editor. The lung: scientific foundations. Philadelphia, Pa: Lippencott-Raven, Inc.; 1997. pp. 371–371. [Google Scholar]
- 69.Sandler A B, Ketner G. Adenovirus early region 4 is essential for normal stability of late nuclear RNAs. J Virol. 1989;63:624–630. doi: 10.1128/jvi.63.2.624-630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sankar S, Mahooti-Brooks N, Centrella M, McCarthy T L, Madri J A. Expression of transforming growth factor type III receptor in vascular endothelial cells increases their responsiveness to transforming growth factor beta 2. J Biol Chem. 1995;270:13567–13572. doi: 10.1074/jbc.270.22.13567. [DOI] [PubMed] [Google Scholar]
- 71.Saris C J, Tack B F, Kristensen T, Glenney J R J, Hunter T. The cDNA sequence for the protein-tyrosine kinase substrate p36 (calpactin I heavy chain) reveals a multidomain protein with internal repeats. Cell. 1986;46:201–212. doi: 10.1016/0092-8674(86)90737-3. [DOI] [PubMed] [Google Scholar]
- 72.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin D B. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990;111:757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Servadio A, Koshy B, Armstrong D, Antalffy B, Orr H T, Zoghbi H Y. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat Genet. 1995;10:94–98. doi: 10.1038/ng0595-94. [DOI] [PubMed] [Google Scholar]
- 74.Shenk T. Adenoviridae: the viruses and their replication. In: Fields B N, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1997. p. 2111. [Google Scholar]
- 75.Spolarics Z, Navarro L. Endotoxin stimulates the expression of glucose-6-phosphate dehydrogenase in Kupffer and hepatic endothelial cells. J Leukoc Biol. 1994;56:453–457. doi: 10.1002/jlb.56.4.453. [DOI] [PubMed] [Google Scholar]
- 76.Tan E M, Uitto J, Bauer E A, Eisen A Z. Human skin fibroblasts in culture: procollagen synthesis in the presence of sera from normal human subjects and from patients with dermal fibroses. J Investig Dermatol. 1981;76:462–467. doi: 10.1111/1523-1747.ep12521119. [DOI] [PubMed] [Google Scholar]
- 77.Toksoz D, Williams D A. Novel human oncogene lbc detected by transfection with distinct homology regions to signal transduction products. Oncogene. 1994;9:621–628. [PubMed] [Google Scholar]
- 78.Verma I M, Somia N. Gene therapy—promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 79.Weller P A, Ogryzko E P, Corben E B, Zhidkova N I, Patel B, Price G J, Spurr N K, Koteliansky V E, Critchley D R. Complete sequence of human vinculin and assignment of the gene to chromosome 10. Proc Natl Acad Sci USA. 1990;87:5667–5671. doi: 10.1073/pnas.87.15.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 81.Wilson J M. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 82.Wrighton C J, Hofer-Warbinek R, Moll T, Eytner R, Bach F H, de Martin R. Inhibition of endothelial cell activation by adenovirus-mediated expression of I kappa B alpha, an inhibitor of the transcription factor NF-kappa B. J Exp Med. 1996;183:1013–1022. doi: 10.1084/jem.183.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeiger M A, Saji M, Caturegli P, Westra W H, Kohn L D, Levine M A. Transformation of rat thyroid follicular cells stably transfected with cholera toxin A1 fragment. Endocrinology. 1996;137:5392–5399. doi: 10.1210/endo.137.12.8940362. [DOI] [PubMed] [Google Scholar]
- 84.Zheng Y, Olson M F, Hall A, Cerione R A, Toksoz D. Direct involvement of the small GTP-binding protein Rho in lbc oncogene function. J Biol Chem. 1995;270:9031–9034. doi: 10.1074/jbc.270.16.9031. [DOI] [PubMed] [Google Scholar]
- 85.Zokas L, Glenney J R J. The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J Cell Biol. 1987;105:2111–2121. doi: 10.1083/jcb.105.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zolotukhin S, Potter M, Hauswirth W W, Guy J, Muzyczka N. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]