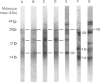
Abstract
Peptide sequence data derived from a plant annexin, P34 [Smallwood, Keen & Bowles (1990) Biochem. J. 270, 157-161] was used to design amplimers for PCR. A unique fragment of 95 bp, amplified from tomato (Lycopersicon esculertum) genomic DNA, was used in Northern analyses and demonstrated a differential pattern of expression in vegetative tissues of tomato, potato (Solanum tuberosum) and barley (Hordeum vulgare). The tissue-specific abundance of the annexin transcript was found to correlate closely with abundance of annexin protein as revealed by their partial purification and analysis with antisera specific for annexins isolated from tomato suspension-culture cells.
Full text
PDF


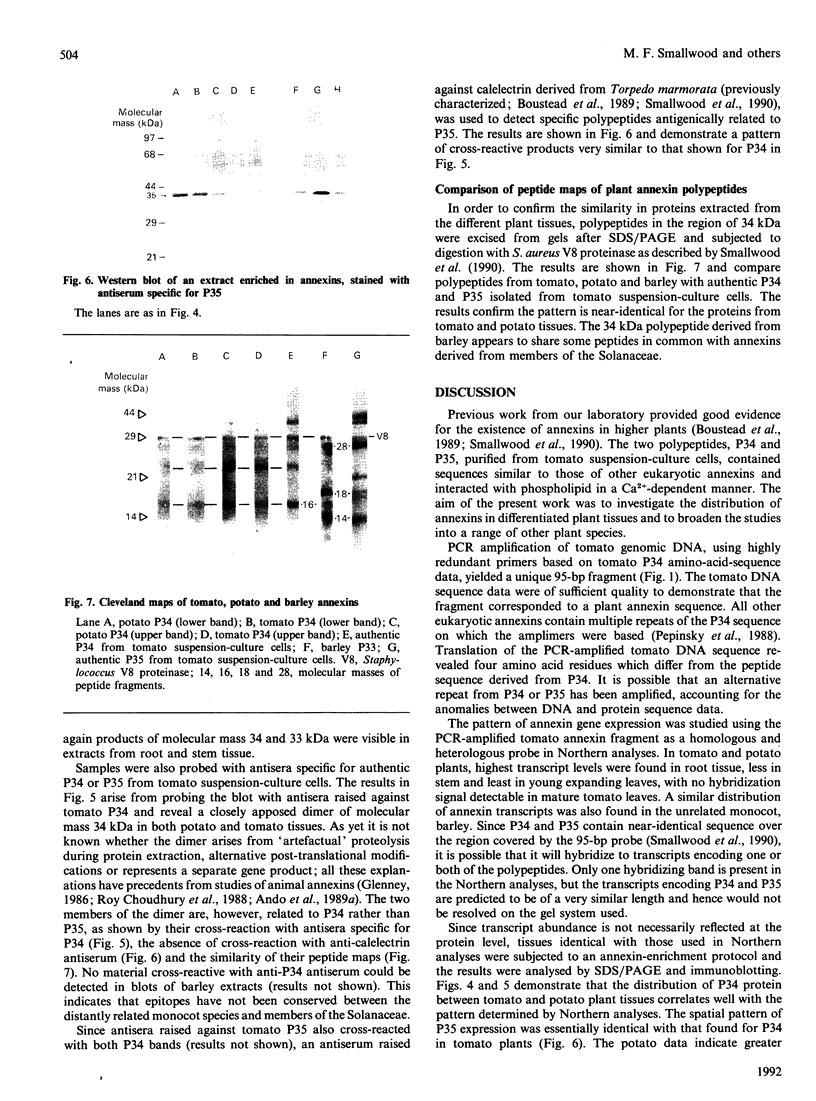

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Ando Y., Imamura S., Hong Y. M., Owada M. K., Kakunaga T., Kannagi R. Enhancement of calcium sensitivity of lipocortin I in phospholipid binding induced by limited proteolysis and phosphorylation at the amino terminus as analyzed by phospholipid affinity column chromatography. J Biol Chem. 1989 Apr 25;264(12):6948–6955. [PubMed] [Google Scholar]
- Ando Y., Imamura S., Owada M. K., Kakunaga T., Kannagi R. Cross-linking of lipocortin I and enhancement of its Ca2+ sensitivity by tissue transglutaminase. Biochem Biophys Res Commun. 1989 Sep 15;163(2):944–951. doi: 10.1016/0006-291x(89)92313-9. [DOI] [PubMed] [Google Scholar]
- Blay J., Valentine-Braun K. A., Northup J. K., Hollenberg M. D. Epidermal-growth-factor-stimulated phosphorylation of calpactin II in membrane vesicles shed from cultured A-431 cells. Biochem J. 1989 Apr 15;259(2):577–583. doi: 10.1042/bj2590577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braslau D. L., Ringo D. L., Rocha V. Synthesis of novel calcium-dependent proteins associated with mammary epithelial cell migration and differentiation. Exp Cell Res. 1984 Nov;155(1):213–221. doi: 10.1016/0014-4827(84)90782-1. [DOI] [PubMed] [Google Scholar]
- Carter C., Howlett A. R., Martin G. S., Bissell M. J. The tyrosine phosphorylation substrate p36 is developmentally regulated in embryonic avian limb and is induced in cell culture. J Cell Biol. 1986 Nov;103(5):2017–2024. doi: 10.1083/jcb.103.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Higgins P., Martin H., Bowles D. J. An embryo-specific protein of barley (Hordeum vulgare). Eur J Biochem. 1991 Jul 1;199(1):115–121. doi: 10.1111/j.1432-1033.1991.tb16098.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. Peptide mapping in one dimension by limited proteolysis of sodium dodecyl sulfate-solubilized proteins. Methods Enzymol. 1983;96:222–229. doi: 10.1016/s0076-6879(83)96020-2. [DOI] [PubMed] [Google Scholar]
- De B. K., Misono K. S., Lukas T. J., Mroczkowski B., Cohen S. A calcium-dependent 35-kilodalton substrate for epidermal growth factor receptor/kinase isolated from normal tissue. J Biol Chem. 1986 Oct 15;261(29):13784–13792. [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Walker J. H., Boustead C., Taylor W. Annexins--new family of Ca2+-regulated-phospholipid binding protein. Biosci Rep. 1987 Apr;7(4):289–298. doi: 10.1007/BF01121450. [DOI] [PubMed] [Google Scholar]
- Giugni T. D., James L. C., Haigler H. T. Epidermal growth factor stimulates tyrosine phosphorylation of specific proteins in permeabilized human fibroblasts. J Biol Chem. 1985 Dec 5;260(28):15081–15090. [PubMed] [Google Scholar]
- Glenney J. R., Jr Calpactins: calcium-regulated membrane-skeletal proteins. Bioessays. 1987 Oct;7(4):173–175. doi: 10.1002/bies.950070408. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Tack B., Powell M. A. Calpactins: two distinct Ca++-regulated phospholipid- and actin-binding proteins isolated from lung and placenta. J Cell Biol. 1987 Mar;104(3):503–511. doi: 10.1083/jcb.104.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. Phospholipid-dependent Ca2+ binding by the 36-kDa tyrosine kinase substrate (calpactin) and its 33-kDa core. J Biol Chem. 1986 Jun 5;261(16):7247–7252. [PubMed] [Google Scholar]
- Gould K. L., Cooper J. A., Hunter T. The 46,000-dalton tyrosine protein kinase substrate is widespread, whereas the 36,000-dalton substrate is only expressed at high levels in certain rodent tissues. J Cell Biol. 1984 Feb;98(2):487–497. doi: 10.1083/jcb.98.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik A., Pepinsky R. B., Shoelson S. E., Kahn C. R. Lipocortins 1 and 2 as substrates for the insulin receptor kinase in rat liver. J Biol Chem. 1988 Aug 25;263(24):11862–11867. [PubMed] [Google Scholar]
- Keutzer J. C., Hirschhorn R. R. The growth-regulated gene 1B6 is identified as the heavy chain of calpactin I. Exp Cell Res. 1990 May;188(1):153–159. doi: 10.1016/0014-4827(90)90291-h. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lozano J. J., Silberstein G. B., Hwang S., Haindl A. H., Rocha V. Developmental regulation of calcium-binding proteins (calelectrins and calpactin I) in mammary glands. J Cell Physiol. 1989 Mar;138(3):503–510. doi: 10.1002/jcp.1041380309. [DOI] [PubMed] [Google Scholar]
- McKanna J. A., Cohen S. The EGF receptor kinase substrate p35 in the floor plate of the embryonic rat CNS. Science. 1989 Mar 17;243(4897):1477–1479. doi: 10.1126/science.2928781. [DOI] [PubMed] [Google Scholar]
- Merril C. R. Gel-staining techniques. Methods Enzymol. 1990;182:477–488. doi: 10.1016/0076-6879(90)82038-4. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K., Chow E. P., O'Brine-Greco B. A dimeric form of lipocortin-1 in human placenta. Biochem J. 1989 Oct 1;263(1):97–103. doi: 10.1042/bj2630097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K. Epidermal growth factor-dependent phosphorylation of lipocortin. Nature. 1986 May 1;321(6065):81–84. doi: 10.1038/321081a0. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Tizard R., Mattaliano R. J., Sinclair L. K., Miller G. T., Browning J. L., Chow E. P., Burne C., Huang K. S., Pratt D. Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem. 1988 Aug 5;263(22):10799–10811. [PubMed] [Google Scholar]
- Roy-Choudhury S., Mishra V. S., Low M. G., Das M. A phospholipid is the membrane-anchoring domain of a protein growth factor of molecular mass 34 kDa in placental trophoblasts. Proc Natl Acad Sci U S A. 1988 Mar;85(6):2014–2018. doi: 10.1073/pnas.85.6.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Miret O. Control of the amount of a 34K Ca2+-dependent membrane binding protein (calelectrin). J Neurochem. 1987 Mar;48(3):745–751. doi: 10.1111/j.1471-4159.1987.tb05580.x. [DOI] [PubMed] [Google Scholar]
- Sawyer S. T., Cohen S. Epidermal growth factor stimulates the phosphorylation of the calcium-dependent 35,000-dalton substrate in intact A-431 cells. J Biol Chem. 1985 Jul 15;260(14):8233–8236. [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. Expression of annexins as a function of cellular growth state. J Cell Biol. 1990 Jul;111(1):229–238. doi: 10.1083/jcb.111.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets E. E., Giugni T. D., Coates G. G., Schlaepfer D. D., Haigler H. T. Epidermal growth factor dependent phosphorylation of a 35-kilodalton protein in placental membranes. Biochemistry. 1987 Feb 24;26(4):1164–1172. doi: 10.1021/bi00378a026. [DOI] [PubMed] [Google Scholar]
- Smallwood M., Keen J. N., Bowles D. J. Purification and partial sequence analysis of plant annexins. Biochem J. 1990 Aug 15;270(1):157–161. doi: 10.1042/bj2700157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V. L., Dedman J. R. An immunological comparison of several novel calcium-binding proteins. J Biol Chem. 1986 Dec 5;261(34):15815–15818. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]