Abstract
Retinal rod-outer-segment phosphodiesterase (PDE) is a heterotetramer consisting of two similar, but not identical, catalytic subunits (alpha and beta) and two identical inhibitory subunits (gamma 2). Previously, we have reported that the site of PDE alpha/beta interaction with PDE gamma is located within residues 54-87 [Cunnick, Hurt, Oppert, Sakamoto & Takemoto (1990) Biochem. J. 271, 721-727]. The site for PDE gamma interaction with transducin alpha (T alpha) was found to encompass residues 24-45 of PDE gamma [Morrison, Cunnick, Oppert & Takemoto (1989) J. Biol. Chem. 264, 11671-11681]. In order to identify binding sites and other functional domains of PDE gamma, the three peptides which are encoded by the three exons of the PDE gamma gene were synthesized chemically. These exons encode for residues 1-49, 50-62 and 63-87 of bovine PDE gamma [Piriev, Purishko, Khramtsov & Lipkin (1990) Dokl. Akad. Nauk. SSSR 315, 229-230]. The peptide encompassing residues 63-87 was inhibitory in a PDE assay, whereas peptides 1-49 and 50-62 had no effect. However, both peptides 1-49 and 63-87 bound to PDE alpha/beta in a solid-phase binding assay. Only peptide 1-49 bound to T alpha.GTP[S] (GTP[S] is guanosine 5'-[gamma-thio]triphosphate). These data confirm that the inhibitory region of PDE gamma is encoded by exon 3 (residues 63-87), whereas a separate binding site for PDE alpha/beta and for T alpha.GTP[S] is encoded by exon 1 (residues 1-49). To study further the structure-function relationship of PDE gamma, this entire protein and two mutants were chemically synthesized. One mutant (-CT) lacked residues 78-87, whereas another replaced tyrosine-84 with glycine (TYR-84). Whereas the synthetic PDE gamma inhibited PDE alpha/beta catalytic activity, the -CT and TVR-84 mutants did not. All three synthetic proteins bound to both PDE alpha/beta and and T alpha.GTP[S]. These data confirm the presence of an alternative binding site on PDE gamma and demonstrate the importance of tyrosine-84 in PDE gamma inhibitory activity.
Full text
PDF


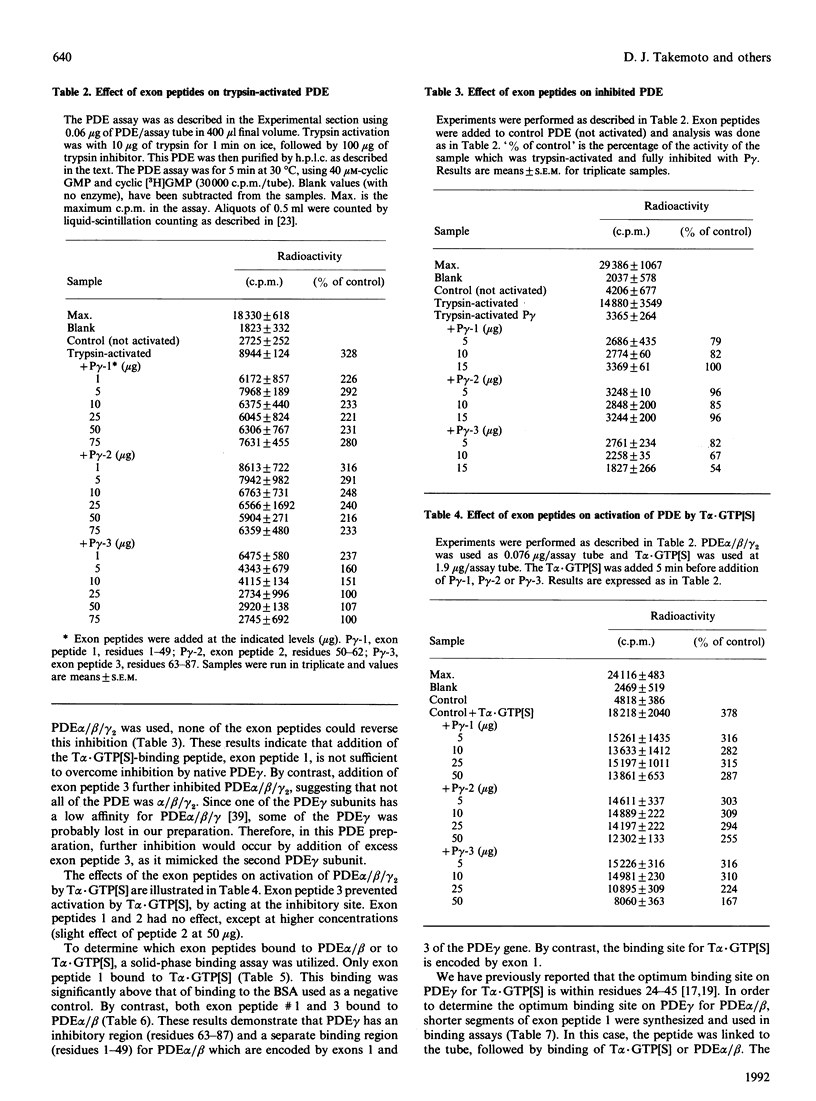

Images in this article
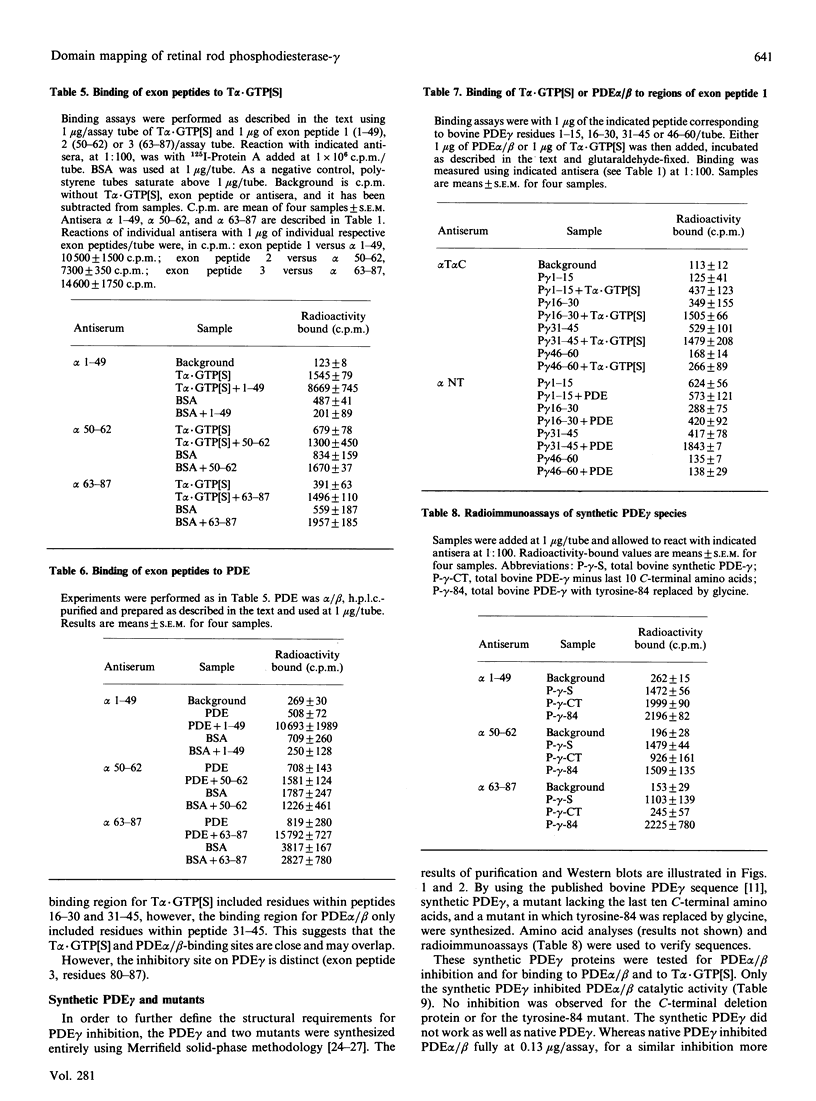
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Hargrave P. A. Molecular biology of the visual pigments. Vision Res. 1986;26(12):1881–1895. doi: 10.1016/0042-6989(86)90115-x. [DOI] [PubMed] [Google Scholar]
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Stryer L. Expression in bacteria of functional inhibitory subunit of retinal rod cGMP phosphodiesterase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4922–4926. doi: 10.1073/pnas.86.13.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M., Deterre P. Molecular mechanism of visual transduction. Eur J Biochem. 1989 Feb 1;179(2):255–266. doi: 10.1111/j.1432-1033.1989.tb14549.x. [DOI] [PubMed] [Google Scholar]
- Cook N. J., Hanke W., Kaupp U. B. Identification, purification, and functional reconstitution of the cyclic GMP-dependent channel from rod photoreceptors. Proc Natl Acad Sci U S A. 1987 Jan;84(2):585–589. doi: 10.1073/pnas.84.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnick J. M., Hurt D., Oppert B., Sakamoto K., Takemoto D. J. Binding of the gamma-subunit of retinal rod-outer-segment phosphodiesterase with both transducin and the catalytic subunits of phosphodiesterase. Biochem J. 1990 Nov 1;271(3):721–727. doi: 10.1042/bj2710721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Forquet F., Robert M., Chabre M. cGMP phosphodiesterase of retinal rods is regulated by two inhibitory subunits. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2424–2428. doi: 10.1073/pnas.85.8.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Robert M., Pfister C., Kühn H., Chabre M. Activation of retinal rod cyclic GMP-phosphodiesterase by transducin: characterization of the complex formed by phosphodiesterase inhibitor and transducin alpha-subunit. Proteins. 1986 Oct;1(2):188–193. doi: 10.1002/prot.340010210. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Griswold-Prenner I. G protein-effector coupling: binding of rod phosphodiesterase inhibitory subunit to transducin. Biochemistry. 1989 Apr 18;28(8):3133–3137. doi: 10.1021/bi00434a003. [DOI] [PubMed] [Google Scholar]
- Gorman J. J. An apparatus for simultaneous manual solid-phase synthesis of multiple peptide analogs. Anal Biochem. 1984 Feb;136(2):397–406. doi: 10.1016/0003-2697(84)90235-5. [DOI] [PubMed] [Google Scholar]
- Griswold-Prenner I., Tuteja N., Farber D. B., Fung B. K. G protein-effector coupling: interactions of recombinant inhibitory gamma subunit with transducin and phosphodiesterase. Biochemistry. 1989 Jul 25;28(15):6145–6150. doi: 10.1021/bi00441a003. [DOI] [PubMed] [Google Scholar]
- Hurley J. B. Molecular properties of the cGMP cascade of vertebrate photoreceptors. Annu Rev Physiol. 1987;49:793–812. doi: 10.1146/annurev.ph.49.030187.004045. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Stryer L. Purification and characterization of the gamma regulatory subunit of the cyclic GMP phosphodiesterase from retinal rod outer segments. J Biol Chem. 1982 Sep 25;257(18):11094–11099. [PubMed] [Google Scholar]
- Hurley J. B., Stryer L. Purification and characterization of the gamma regulatory subunit of the cyclic GMP phosphodiesterase from retinal rod outer segments. J Biol Chem. 1982 Sep 25;257(18):11094–11099. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Parker K. R., Dratz E. A. The molecular mechanism of visual excitation and its relation to the structure and composition of the rod outer segment. Annu Rev Physiol. 1987;49:765–791. doi: 10.1146/annurev.ph.49.030187.004001. [DOI] [PubMed] [Google Scholar]
- Lipkin V. M., Khramtsov N. V., Vasilevskaya I. A., Atabekova N. V., Muradov K. G., Gubanov V. V., Li T., Johnston J. P., Volpp K. J., Applebury M. L. Beta-subunit of bovine rod photoreceptor cGMP phosphodiesterase. Comparison with the phosphodiesterase family. J Biol Chem. 1990 Aug 5;265(22):12955–12959. [PubMed] [Google Scholar]
- Lookhart G. L., Jones B. L., Cooper D. B., Hall S. B. A method for hydrolyzing and determining the amino acid compositions of picomole quantities of proteins in less than 3 hours. J Biochem Biophys Methods. 1982 Dec;7(1):15–23. doi: 10.1016/0165-022x(82)90032-x. [DOI] [PubMed] [Google Scholar]
- Medynski D. C., Sullivan K., Smith D., Van Dop C., Chang F. H., Fung B. K., Seeburg P. H., Bourne H. R. Amino acid sequence of the alpha subunit of transducin deduced from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4311–4315. doi: 10.1073/pnas.82.13.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. F., Cunnick J. M., Oppert B., Takemoto D. J. Interaction of the gamma-subunit of retinal rod outer segment phosphodiesterase with transducin. Use of synthetic peptides as functional probes. J Biol Chem. 1989 Jul 15;264(20):11671–11681. [PubMed] [Google Scholar]
- Morrison D. F., Rider M. A., Takemoto D. J. Modulation of retinal transducin and phosphodiesterase activities by synthetic peptides of the phosphodiesterase gamma-subunit. FEBS Lett. 1987 Oct 5;222(2):266–270. doi: 10.1016/0014-5793(87)80383-6. [DOI] [PubMed] [Google Scholar]
- Nathans J. Molecular biology of visual pigments. Annu Rev Neurosci. 1987;10:163–194. doi: 10.1146/annurev.ne.10.030187.001115. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Gubanov V. V., Khramtsov N. V., Ischenko K. A., Zagranichny V. E., Muradov K. G., Shuvaeva T. M., Lipkin V. M. Cyclic GMP phosphodiesterase from bovine retina. Amino acid sequence of the alpha-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1987 Oct 19;223(1):169–173. doi: 10.1016/0014-5793(87)80530-6. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Lipkin V. M., Kumarev V. P., Gubanov V. V., Khramtsov N. V., Akhmedov N. B., Zagranichny V. E., Muradov K. G. Cyclic GMP phosphodiesterase from cattle retina. Amino acid sequence of the gamma-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1986 Aug 18;204(2):288–292. doi: 10.1016/0014-5793(86)80830-4. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Piriev N. I., Purishko V. A., Khramtsov N. V., Lipkin V. M. Organizatsiia gena gamma-sub''edinitsy fotoretseptornoi fosfodiésterazy tsiklicheskogo GMF cheloveka. Dokl Akad Nauk SSSR. 1990;315(1):229–231. [PubMed] [Google Scholar]
- Pugh E. N., Jr The nature and identity of the internal excitational transmitter of vertebrate phototransduction. Annu Rev Physiol. 1987;49:715–741. doi: 10.1146/annurev.ph.49.030187.003435. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Suter M. A modified ELISA technique for anti-hapten antibodies. J Immunol Methods. 1982 Aug 27;53(1):103–108. doi: 10.1016/0022-1759(82)90244-7. [DOI] [PubMed] [Google Scholar]
- Takemoto D. J., Spooner B., Takemoto L. J. Antisera to synthetic peptides of bovine rhodopsin: use as site-specific probes of disc membrane changes in retinal dystrophic dogs. Biochem Biophys Res Commun. 1985 Oct 15;132(1):438–444. doi: 10.1016/0006-291x(85)91041-1. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Tuteja N., Farber D. B. Gamma-subunit of mouse retinal cyclic-GMP phosphodiesterase: cDNA and corresponding amino acid sequence. FEBS Lett. 1988 May 9;232(1):182–186. doi: 10.1016/0014-5793(88)80413-7. [DOI] [PubMed] [Google Scholar]
- Whalen M. M., Bitensky M. W. Comparison of the phosphodiesterase inhibitory subunit interactions of frog and bovine rod outer segments. Biochem J. 1989 Apr 1;259(1):13–19. doi: 10.1042/bj2590013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen M. M., Bitensky M. W., Takemoto D. J. The effect of the gamma-subunit of the cyclic GMP phosphodiesterase of bovine and frog (Rana catesbiana) retinal rod outer segments on the kinetic parameters of the enzyme. Biochem J. 1990 Feb 1;265(3):655–658. doi: 10.1042/bj2650655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki A., Bartucca F., Ting A., Bitensky M. W. Reciprocal effects of an inhibitory factor on catalytic activity and noncatalytic cGMP binding sites of rod phosphodiesterase. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3702–3706. doi: 10.1073/pnas.79.12.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki A., Stein P. J., Chernoff N., Bitensky M. W. Activation mechanism of rod outer segment cyclic GMP phosphodiesterase. Release of inhibitor by the GTP/GTP-binding protein. J Biol Chem. 1983 Jul 10;258(13):8188–8194. [PubMed] [Google Scholar]