Abstract
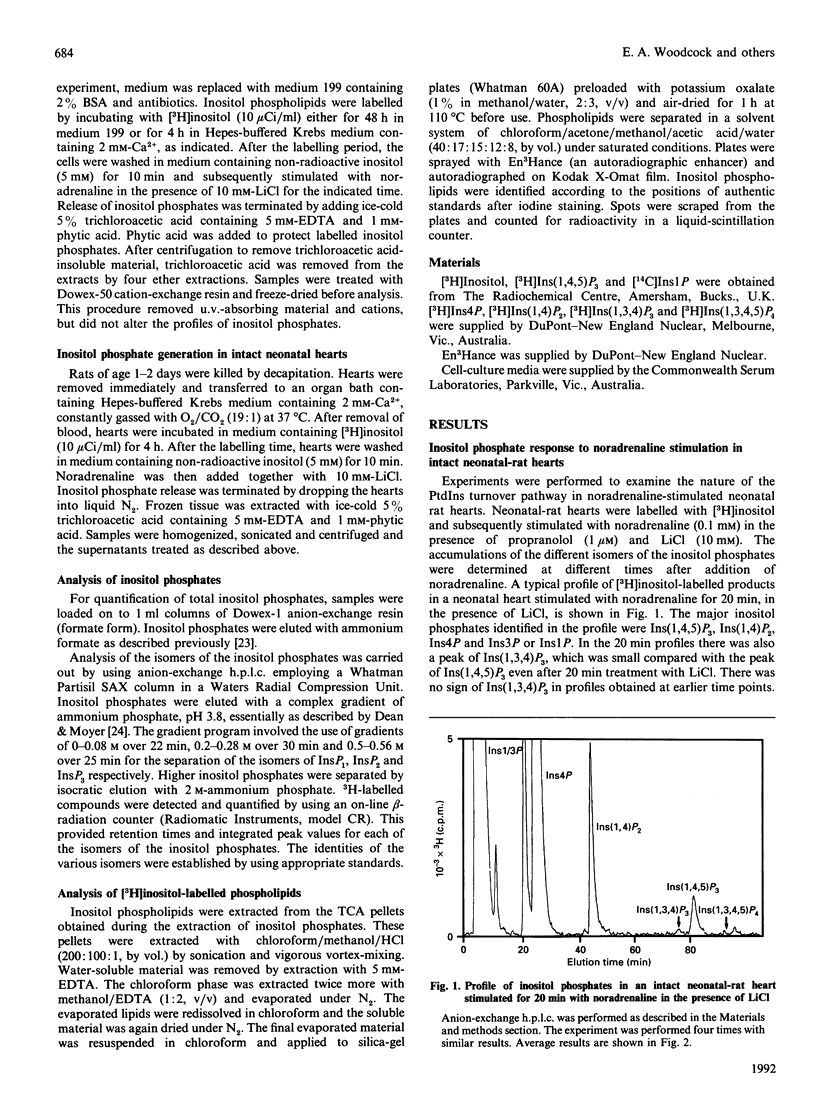
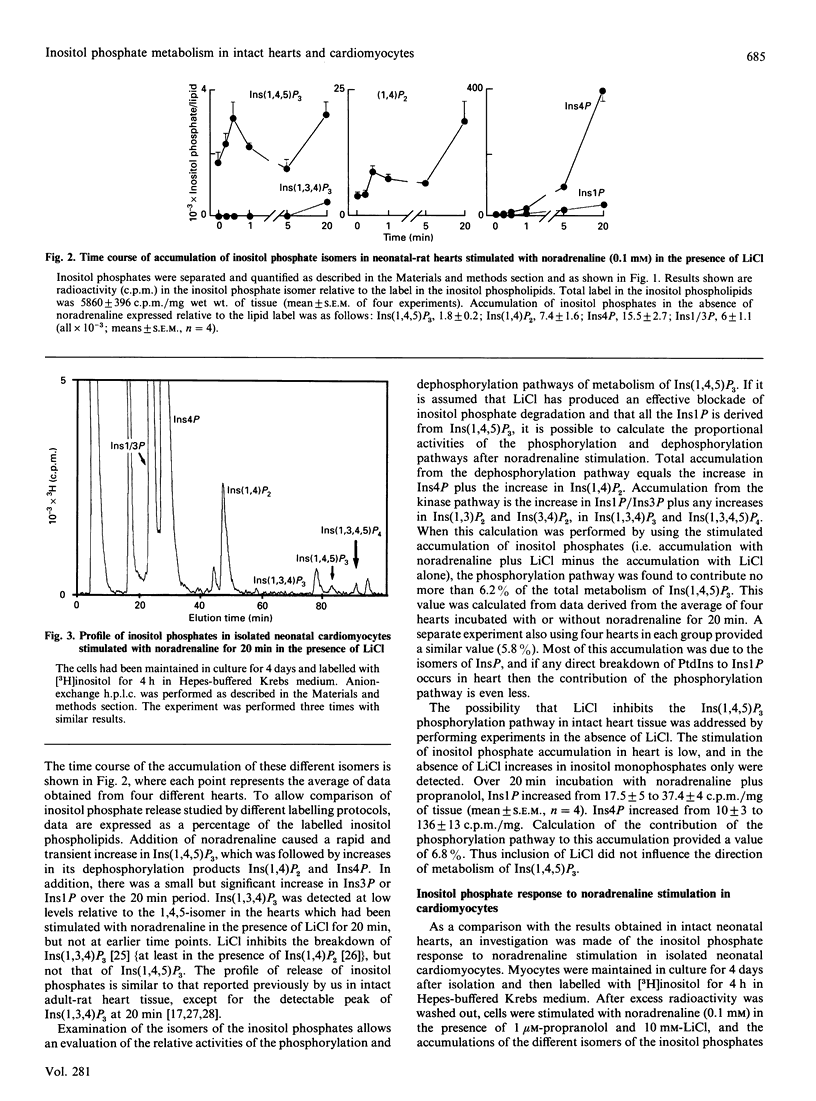
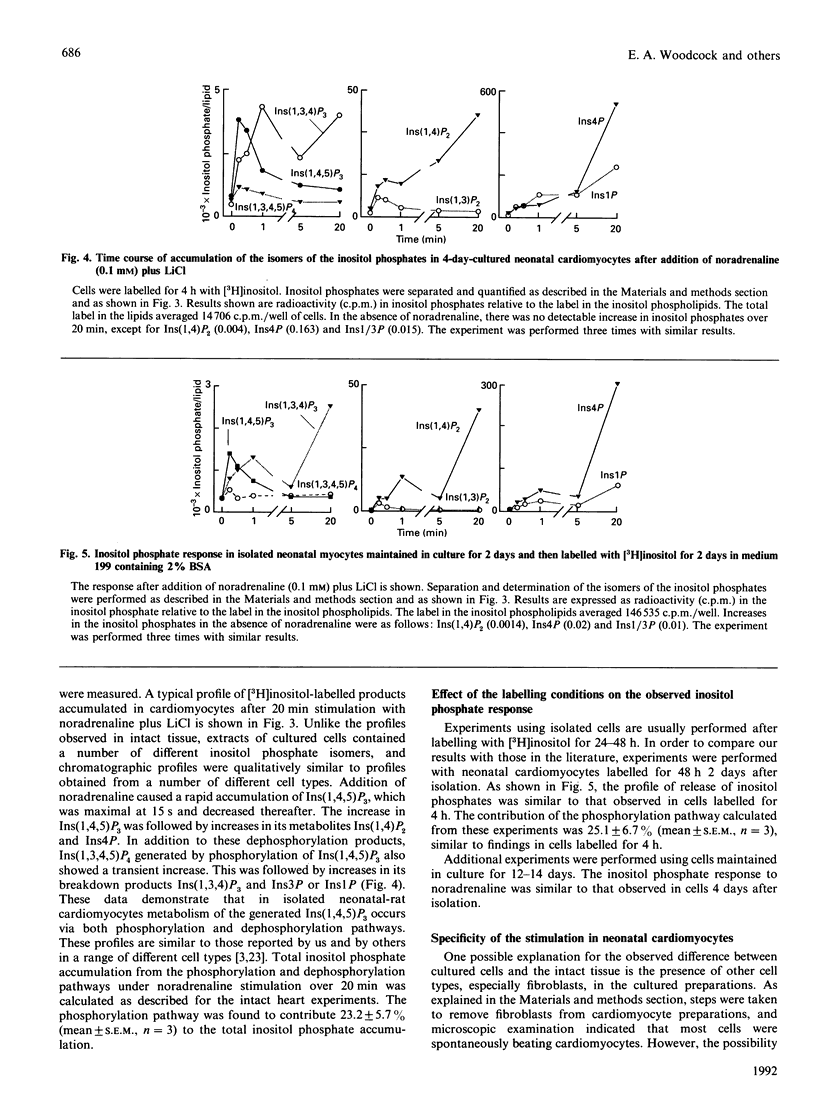
In most tissues stimulation of the phosphatidylinositol turnover pathway causes release of inositol 1,4,5-trisphosphate [Ins(1,4,5)P3], which is subsequently metabolized to a wide range of inositol phosphate isomers deriving from both phosphorylation and dephosphorylation reactions. However, addition of noradrenaline to isolated intact neonatal-rat hearts generated only those inositol phosphates produced by dephosphorylation of Ins(1,4,5)P3. Products of the InsP3 kinase pathway were absent from the profiles, except after prolonged stimulation. In contrast, addition of noradrenaline to isolated cultured neonatal-rat cardiomyocytes caused the release of Ins(1,4,5)P3, which was metabolized by both phosphorylation and dephosphorylation pathways to yield a complex range of inositol phosphate isomers, as observed in many other cell types. These differences between the responses in intact tissues and in isolated cell preparations were not caused by the different conditions used for [3H]inositol labelling. Furthermore, results could not be explained by overgrowth of other cell types in the isolated cell preparations. Thus the results demonstrate that the isolation and culture of rat neonatal cardiomyocytes produces alterations in the nature of the phosphatidylinositol turnover pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar N., Nambi P., Stassen F. L., Crooke S. T. Vascular vasopressin receptors mediate phosphatidylinositol turnover and calcium efflux in an established smooth muscle cell line. Life Sci. 1986 Jul 7;39(1):37–45. doi: 10.1016/0024-3205(86)90435-2. [DOI] [PubMed] [Google Scholar]
- Ambler S. K., Thompson B., Solski P. A., Brown J. H., Taylor P. Receptor-mediated inositol phosphate formation in relation to calcium mobilization: a comparison of two cell lines. Mol Pharmacol. 1987 Sep;32(3):376–383. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bird G. J., Oliver K. G., Horstman D. A., Obie J., Putney J. W., Jr Relationship between the calcium-mobilizing action of inositol 1,4,5-trisphosphate in permeable AR4-2J cells and the estimated levels of inositol 1,4,5-trisphosphate in intact AR4-2J cells. Biochem J. 1991 Feb 1;273(Pt 3):541–546. doi: 10.1042/bj2730541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Rudnick M., Letcher A. J., Irvine R. F. Formation of methylphosphoryl inositol phosphates by extractions that employ methanol. Biochem J. 1988 Aug 1;253(3):703–710. doi: 10.1042/bj2530703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Buxton I. L., Brunton L. L. Alpha 1-adrenergic and muscarinic cholinergic stimulation of phosphoinositide hydrolysis in adult rat cardiomyocytes. Circ Res. 1985 Oct;57(4):532–537. doi: 10.1161/01.res.57.4.532. [DOI] [PubMed] [Google Scholar]
- De Smedt H., Parys J. B., Himpens B., Missiaen L., Borghgraef R. Changes in the mechanism of Ca2(+) mobilization during the differentiation of BC3H1 muscle cells. Biochem J. 1991 Jan 1;273(Pt 1):219–223. doi: 10.1042/bj2730219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Separation of multiple isomers of inositol phosphates formed in GH3 cells. Biochem J. 1987 Mar 1;242(2):361–366. doi: 10.1042/bj2420361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A., Roldan E. R., Lander D. J., Irvine R. F. Ram spermatozoa produce inositol 1,4,5-trisphosphate but not inositol 1,3,4,5-tetrakisphosphate during the Ca2+/ionophore-induced acrosome reaction. Cell Signal. 1990;2(3):277–284. doi: 10.1016/0898-6568(90)90055-f. [DOI] [PubMed] [Google Scholar]
- Horstman D. A., Takemura H., Putney J. W., Jr Formation and metabolism of [3H]inositol phosphates in AR42J pancreatoma cells. Substance P-induced Ca2+ mobilization in the apparent absence of inositol 1,4,5-trisphosphate 3-kinase activity. J Biol Chem. 1988 Oct 25;263(30):15297–15303. [PubMed] [Google Scholar]
- Hughes P. J., Drummond A. H. Formation of inositol phosphate isomers in GH3 pituitary tumour cells stimulated with thyrotropin-releasing hormone. Acute effects of lithium ions. Biochem J. 1987 Dec 1;248(2):463–470. doi: 10.1042/bj2480463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H., Iizuka K., Takahashi H., Yasuda H. Inositol trisphosphate kinase activity in hypertrophied rat heart. Biochem Med Metab Biol. 1990 Aug;44(1):42–50. doi: 10.1016/0885-4505(90)90043-z. [DOI] [PubMed] [Google Scholar]
- Keller A. M., Clancy R. M., Barr M. L., Marboe C. C., Cannon P. J. Acute reoxygenation injury in the isolated rat heart: role of resident cardiac mast cells. Circ Res. 1988 Dec;63(6):1044–1052. doi: 10.1161/01.res.63.6.1044. [DOI] [PubMed] [Google Scholar]
- Kelly R. A., Eid H., Krämer B. K., O'Neill M., Liang B. T., Reers M., Smith T. W. Endothelin enhances the contractile responsiveness of adult rat ventricular myocytes to calcium by a pertussis toxin-sensitive pathway. J Clin Invest. 1990 Oct;86(4):1164–1171. doi: 10.1172/JCI114822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C., Barsotti R. J., Lea T. J., Mulligan I. P., Patel J. R., Ferenczi M. A. Calcium release from cardiac sarcoplasmic reticulum induced by photorelease of calcium or Ins(1,4,5)P3. Am J Physiol. 1990 Feb;258(2 Pt 2):H610–H615. doi: 10.1152/ajpheart.1990.258.2.H610. [DOI] [PubMed] [Google Scholar]
- Kohl C., Schmitz W., Scholz H., Scholz J. Evidence for the existence of inositol tetrakisphosphate in mammalian heart. Effect of alpha 1-adrenoceptor stimulation. Circ Res. 1990 Feb;66(2):580–583. doi: 10.1161/01.res.66.2.580. [DOI] [PubMed] [Google Scholar]
- Kuraja I. J., Tanner J. K., Woodcock E. A. Endothelin stimulates phosphatidylinositol turnover in rat right and left atria. Eur J Pharmacol. 1990 Oct 30;189(4-5):299–306. doi: 10.1016/0922-4106(90)90123-f. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Rampe D., Brown A. M. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988 Sep 15;335(6187):249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- Lambert D. G., Challiss R. A., Nahorski S. R. Accumulation and metabolism of Ins(1,4,5)P3 and Ins(1,3,4,5)P4 in muscarinic-receptor-stimulated SH-SY5Y neuroblastoma cells. Biochem J. 1991 Feb 1;273(Pt 3):791–794. doi: 10.1042/bj2730791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee E. E., Clark M. G., Beinlich C. J., Lins J. A., Morgan H. E. Neutral-alkaline proteases and protein degradation in rat heart. J Mol Cell Cardiol. 1979 Oct;11(10):1033–1051. doi: 10.1016/0022-2828(79)90393-6. [DOI] [PubMed] [Google Scholar]
- Movsesian M. A., Thomas A. P., Selak M., Williamson J. R. Inositol trisphosphate does not release Ca2+ from permeabilized cardiac myocytes and sarcoplasmic reticulum. FEBS Lett. 1985 Jun 17;185(2):328–332. doi: 10.1016/0014-5793(85)80932-7. [DOI] [PubMed] [Google Scholar]
- Nosek T. M., Williams M. F., Zeigler S. T., Godt R. E. Inositol trisphosphate enhances calcium release in skinned cardiac and skeletal muscle. Am J Physiol. 1986 May;250(5 Pt 1):C807–C811. doi: 10.1152/ajpcell.1986.250.5.C807. [DOI] [PubMed] [Google Scholar]
- Otani H., Otani H., Das D. K. Alpha 1-adrenoceptor-mediated phosphoinositide breakdown and inotropic response in rat left ventricular papillary muscles. Circ Res. 1988 Jan;62(1):8–17. doi: 10.1161/01.res.62.1.8. [DOI] [PubMed] [Google Scholar]
- Renard D., Poggioli J. Does the inositol tris/tetrakisphosphate pathway exist in rat heart? FEBS Lett. 1987 Jun 8;217(1):117–123. doi: 10.1016/0014-5793(87)81254-1. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Gross S. S., Levi R. Positive inotropic effect of histamine on guinea pig left atrium: H1-receptor-induced stimulation of phosphoinositide turnover. J Pharmacol Exp Ther. 1988 Nov;247(2):466–472. [PubMed] [Google Scholar]
- Scholz J., Schaefer B., Schmitz W., Scholz H., Steinfath M., Lohse M., Schwabe U., Puurunen J. Alpha-1 adrenoceptor-mediated positive inotropic effect and inositol trisphosphate increase in mammalian heart. J Pharmacol Exp Ther. 1988 Apr;245(1):327–335. [PubMed] [Google Scholar]
- Shears S. B. Metabolism of the inositol phosphates produced upon receptor activation. Biochem J. 1989 Jun 1;260(2):313–324. doi: 10.1042/bj2600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B., Storey D. J., Morris A. J., Cubitt A. B., Parry J. B., Michell R. H., Kirk C. J. Dephosphorylation of myo-inositol 1,4,5-trisphosphate and myo-inositol 1,3,4-triphosphate. Biochem J. 1987 Mar 1;242(2):393–402. doi: 10.1042/bj2420393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res. 1985 Jun;56(6):884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- Steinberg S. F., Kaplan L. M., Inouye T., Zhang J. F., Robinson R. B. Alpha-1 adrenergic stimulation of 1,4,5-inositol trisphosphate formation in ventricular myocytes. J Pharmacol Exp Ther. 1989 Sep;250(3):1141–1148. [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Weissberg P. L., Little P. J., Cragoe E. J., Jr, Bobik A. The pH of spontaneously beating cultured rat heart cells is regulated by an ATP-calmodulin-dependent Na+/H+ antiport. Circ Res. 1989 Apr;64(4):676–685. doi: 10.1161/01.res.64.4.676. [DOI] [PubMed] [Google Scholar]
- Woodcock E. A., Little P. J., Tanner J. K. Inositol phosphate release and steroidogenesis in rat adrenal glomerulosa cells. Comparison of the effects of endothelin, angiotensin II and vasopressin. Biochem J. 1990 Nov 1;271(3):791–796. doi: 10.1042/bj2710791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock E. A., Smith A. I., Wallace C. A., White L. B. Evidence for a lack of inositol--(1,4,5)trisphosphate kinase activity in norepinephrine-perfused rat hearts. Biochem Biophys Res Commun. 1987 Oct 14;148(1):68–77. doi: 10.1016/0006-291x(87)91077-1. [DOI] [PubMed] [Google Scholar]
- Woodcock E. A., White L. B., Smith A. I., McLeod J. K. Stimulation of phosphatidylinositol metabolism in the isolated, perfused rat heart. Circ Res. 1987 Nov;61(5):625–631. doi: 10.1161/01.res.61.5.625. [DOI] [PubMed] [Google Scholar]
- Yuan S. H., Sunahara F. A., Sen A. K. Tumor-promoting phorbol esters inhibit cardiac functions and induce redistribution of protein kinase C in perfused beating rat heart. Circ Res. 1987 Sep;61(3):372–378. doi: 10.1161/01.res.61.3.372. [DOI] [PubMed] [Google Scholar]
- von Harsdorf R., Lang R. E., Fullerton M., Woodcock E. A. Myocardial stretch stimulates phosphatidylinositol turnover. Circ Res. 1989 Aug;65(2):494–501. doi: 10.1161/01.res.65.2.494. [DOI] [PubMed] [Google Scholar]
- von Harsdorf R., Lang R., Fullerton M., Smith A. I., Woodcock E. A. Right atrial dilatation increases inositol-(1,4,5)trisphosphate accumulation. Implications for the control of atrial natriuretic peptide release. FEBS Lett. 1988 Jun 6;233(1):201–205. doi: 10.1016/0014-5793(88)81384-x. [DOI] [PubMed] [Google Scholar]


