Abstract
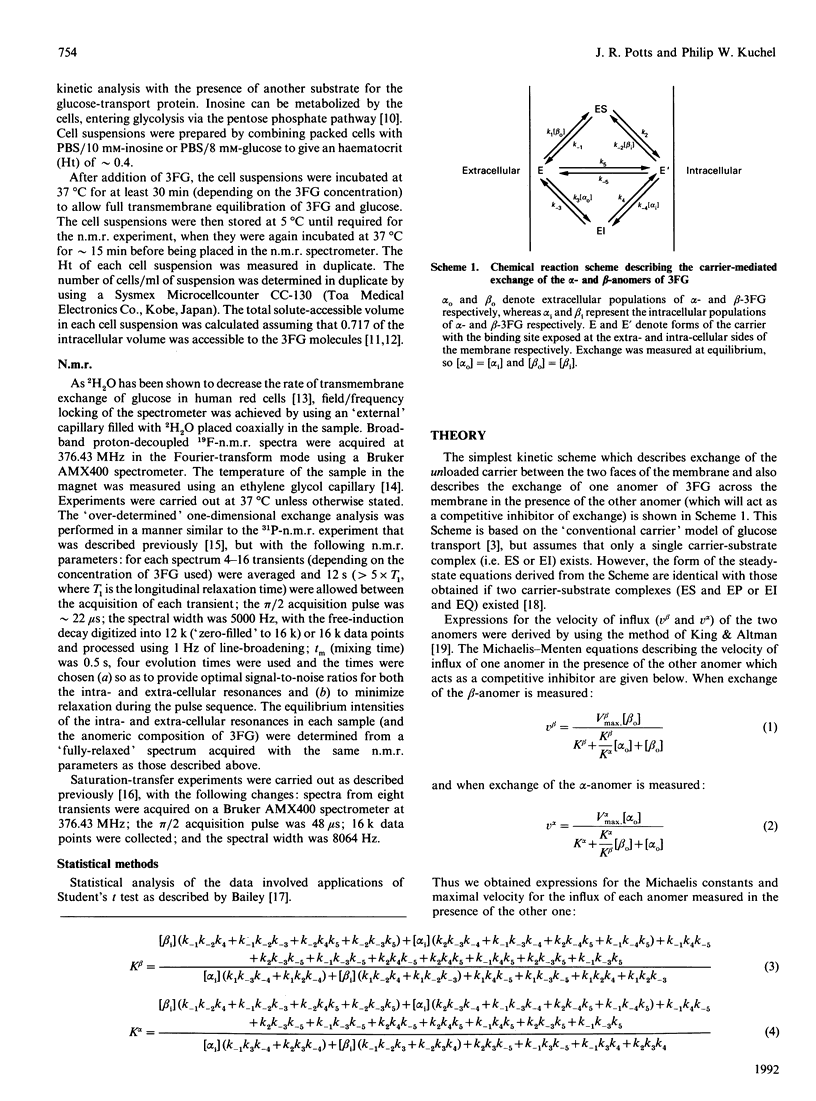
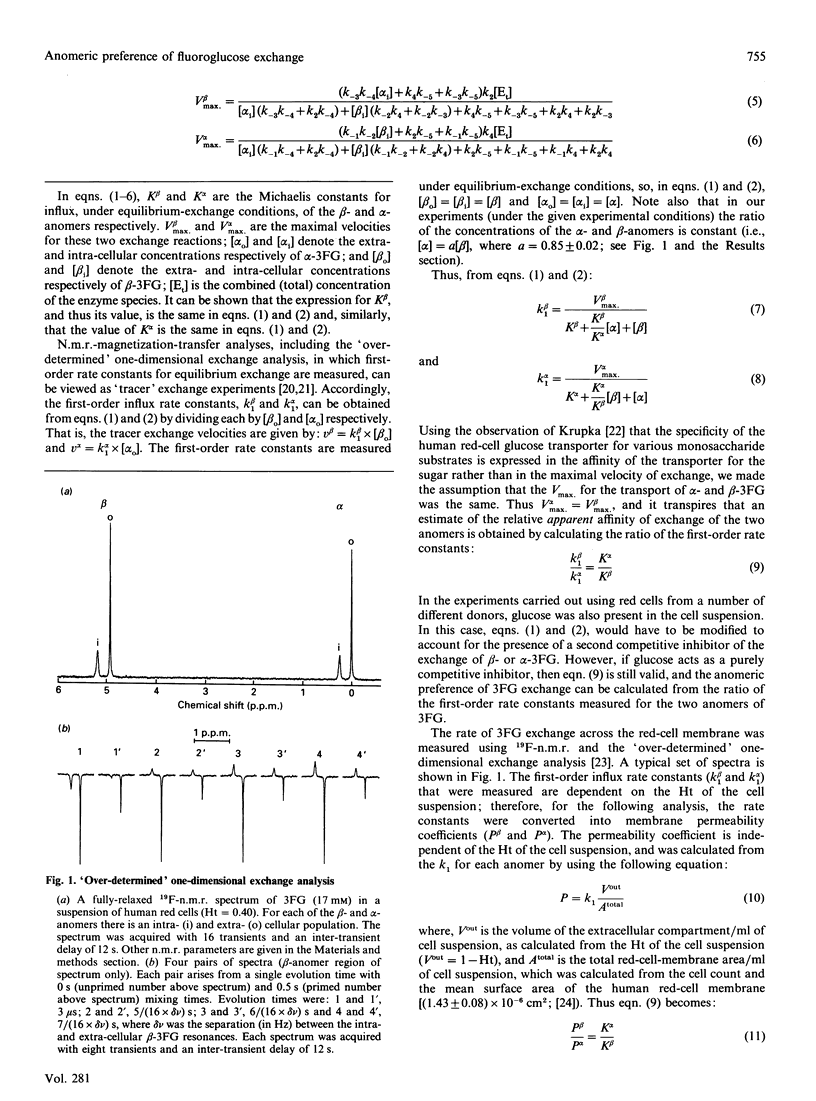
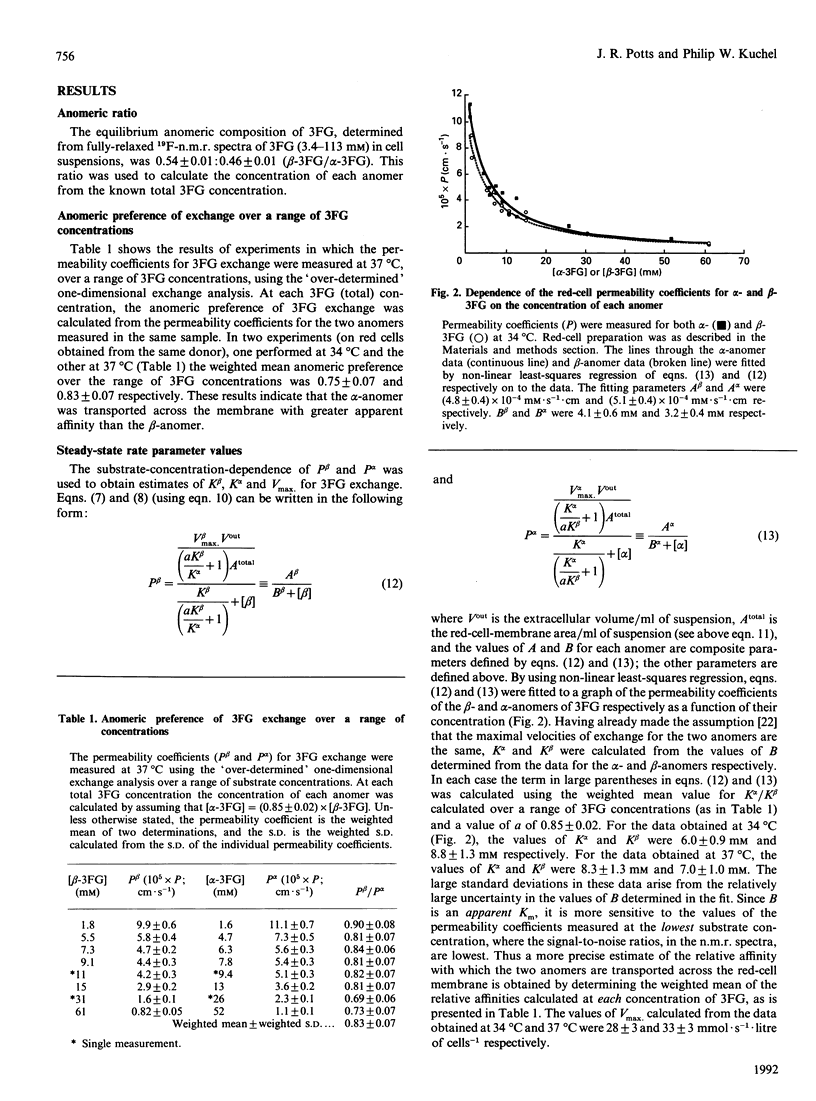
The rates of exchange across the human red-cell membrane of the alpha- and beta-anomers of the glucose derivative 3-fluoro-3-deoxy-D-glucose (3FG) were measured, under equilibrium-exchange conditions, using a 19F-n.m.r.-magnetization-exchange procedure. In experiments carried out over a range of 3FG concentrations (3.4-113 mM), the alpha-anomer was found to be transported with a smaller Km (greater apparent affinity) than the beta-anomer. In two experiments carried out at 34 and 37 degrees C the ratio (alpha/beta) of the Michaelis constants for exchange was 0.75 +/- 0.07 and 0.83 +/- 0.07 respectively and the Vmax for 3FG exchange was 28 +/- 3 and 33 +/- 3 mmol.s-1.litre of cells-1 respectively. In several experiments carried out at a single 3FG concentration (17 mM) and at 37 degrees C, using red cells from four individuals, the rate of exchange of the alpha-anomer across the membrane was significantly higher than that of the beta-anomer. The weighted mean value of the above-mentioned ratio was 0.79 +/- 0.07 for the four donors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleman J. R., Lienhard G. E. Kinetics of the purified glucose transporter. Direct measurement of the rates of interconversion of transporter conformers. Biochemistry. 1989 Oct 3;28(20):8221–8227. doi: 10.1021/bi00446a038. [DOI] [PubMed] [Google Scholar]
- Baker G. F., Naftalin R. J. The effects of replacement of water with D2O on D-glucose transfer in human erythrocytes [proceedings]. J Physiol. 1978 Jul;280:25P–25P. [PubMed] [Google Scholar]
- Baldwin S. A., Baldwin J. M., Lienhard G. E. Monosaccharide transporter of the human erythrocyte. Characterization of an improved preparation. Biochemistry. 1982 Aug 3;21(16):3836–3842. doi: 10.1021/bi00259a018. [DOI] [PubMed] [Google Scholar]
- Barnett J. E., Holman G. D., Munday K. A. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem J. 1973 Feb;131(2):211–221. doi: 10.1042/bj1310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo C. M. Nonsolvent water in human erythrocytes and hemoglobin solutions. J Gen Physiol. 1967 Dec;50(11):2547–2564. doi: 10.1085/jgp.50.11.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers A., Melchior D. L. Transport of alpha- and beta-D-glucose by the intact human red cell. Biochemistry. 1985 Jul 16;24(15):4244–4250. doi: 10.1021/bi00336a065. [DOI] [PubMed] [Google Scholar]
- FAUST R. G. Monosaccharide penetration into human red blood cells by an altered diffusion mechanism. J Cell Comp Physiol. 1960 Oct;56:103–121. doi: 10.1002/jcp.1030560205. [DOI] [PubMed] [Google Scholar]
- Fujii H., Miwa I., Okuda J., Tamura A., Fujii T. Glucose transport into human erythrocytes treated with phospholipase A2 or C. Biochim Biophys Acta. 1986 Aug 6;883(1):77–82. doi: 10.1016/0304-4165(86)90137-6. [DOI] [PubMed] [Google Scholar]
- Gasbjerg P. K., Brahm J. Glucose transport kinetics in human red blood cells. Biochim Biophys Acta. 1991 Feb 11;1062(1):83–93. doi: 10.1016/0005-2736(91)90338-9. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Lienhard G. E. Equilibria and kinetics of ligand binding to the human erythrocyte glucose transporter. Evidence for an alternating conformation model for transport. Biochemistry. 1981 Sep 1;20(18):5108–5113. doi: 10.1021/bi00521a003. [DOI] [PubMed] [Google Scholar]
- Krupka R. M. Expression of substrate specificity in facilitated transport systems. J Membr Biol. 1990 Jul;117(1):69–78. doi: 10.1007/BF01871566. [DOI] [PubMed] [Google Scholar]
- Kuchel P. W., Chapman B. E. NMR spin exchange kinetics at equilibrium in membrane transport and enzyme systems. J Theor Biol. 1983 Dec 21;105(4):569–589. doi: 10.1016/0022-5193(83)90220-5. [DOI] [PubMed] [Google Scholar]
- Kuchel P. W. Steady-state parameters of an enzyme from n.m.r. spin transfer with thermal variation. Biochem J. 1987 May 15;244(1):247–248. doi: 10.1042/bj2440247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEVRE P. G., MARSHALL J. K. Conformational specificity in a biological sugar transport system. Am J Physiol. 1958 Aug;194(2):333–337. doi: 10.1152/ajplegacy.1958.194.2.333. [DOI] [PubMed] [Google Scholar]
- Lowe A. G., Walmsley A. R. The kinetics of glucose transport in human red blood cells. Biochim Biophys Acta. 1986 May 28;857(2):146–154. doi: 10.1016/0005-2736(86)90342-1. [DOI] [PubMed] [Google Scholar]
- Potts J. R., Hounslow A. M., Kuchel P. W. Exchange of fluorinated glucose across the red-cell membrane measured by 19F-n.m.r. magnetization transfer. Biochem J. 1990 Mar 15;266(3):925–928. [PMC free article] [PubMed] [Google Scholar]
- Price W. S., Kuchel P. W. Hypophosphite transport in human erythrocytes studied by overdetermined one-dimensional NMR exchange analysis. NMR Biomed. 1990 Apr;3(2):59–63. doi: 10.1002/nbm.1940030203. [DOI] [PubMed] [Google Scholar]
- Riley G. J., Taylor N. F. The interaction of 3-deoxy-3-fluoro-D-glucose with the hexose-transport system of the human erythrocyte. Biochem J. 1973 Dec;135(4):773–777. doi: 10.1042/bj1350773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVITZ D., SIDEL V. W., SOLOMON A. K. OSMOTIC PROPERTIES OF HUMAN RED CELLS. J Gen Physiol. 1964 Sep;48:79–94. doi: 10.1085/jgp.48.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]


