Abstract
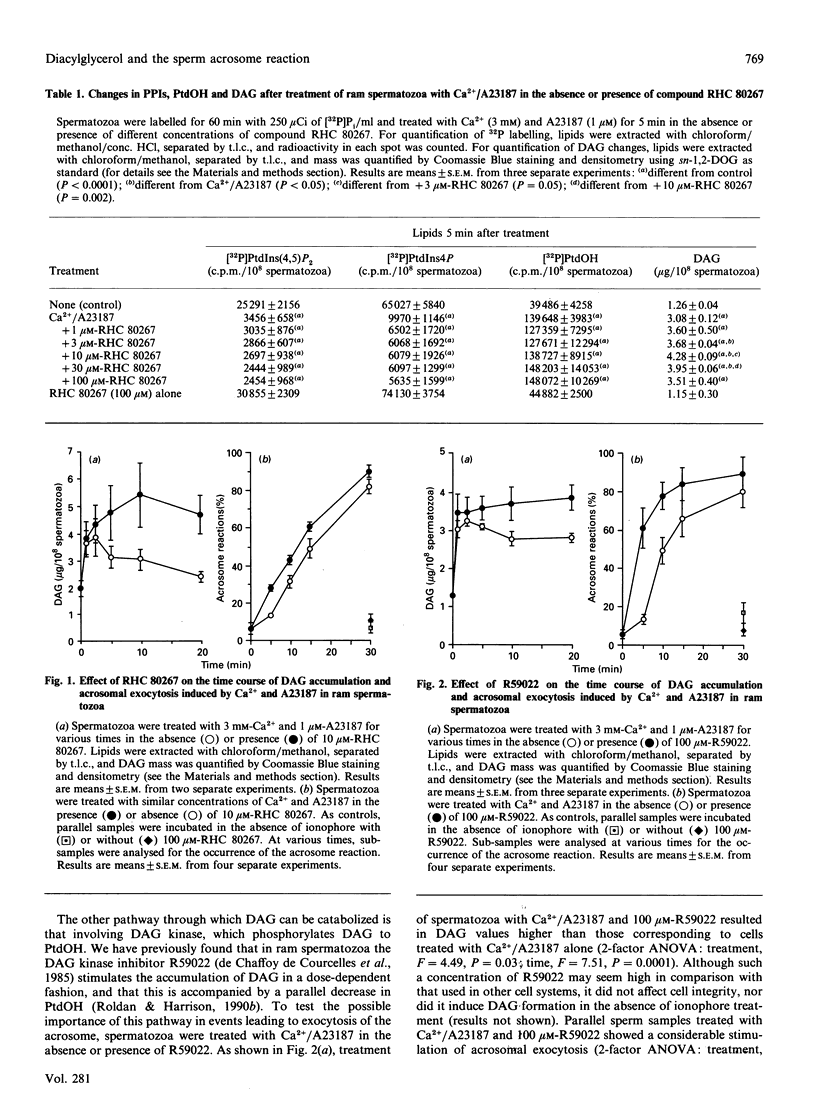
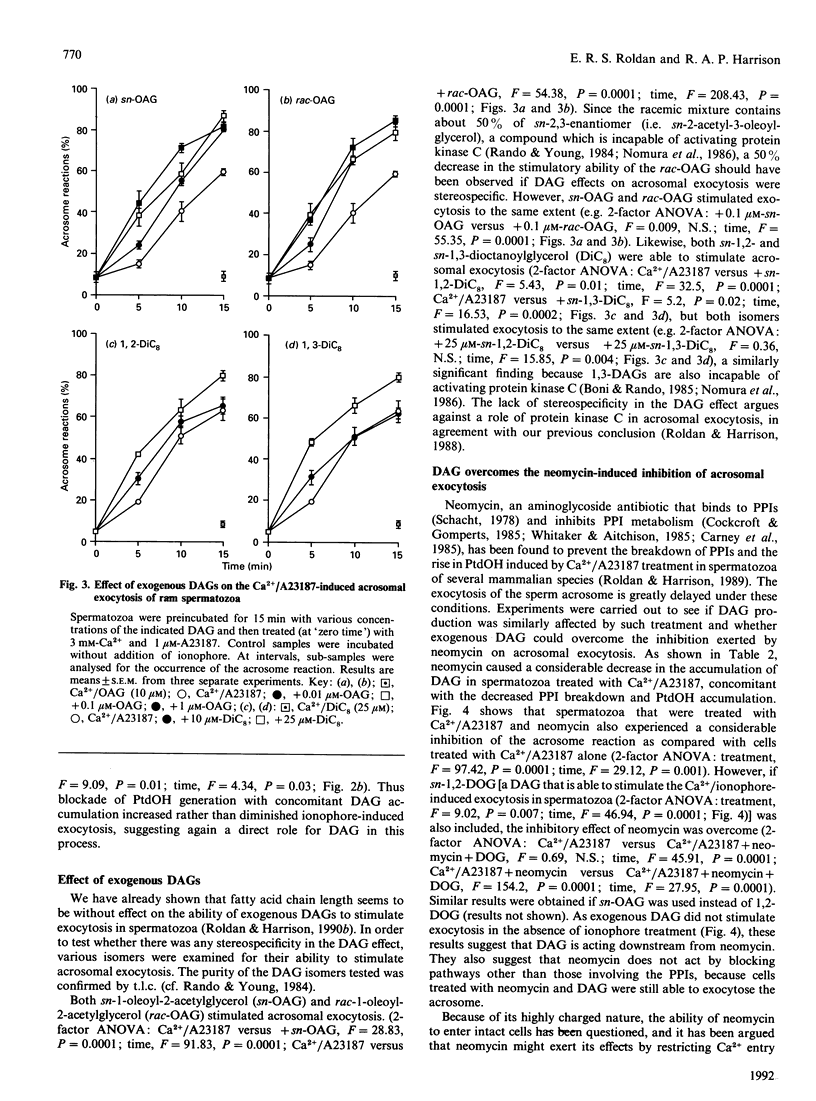
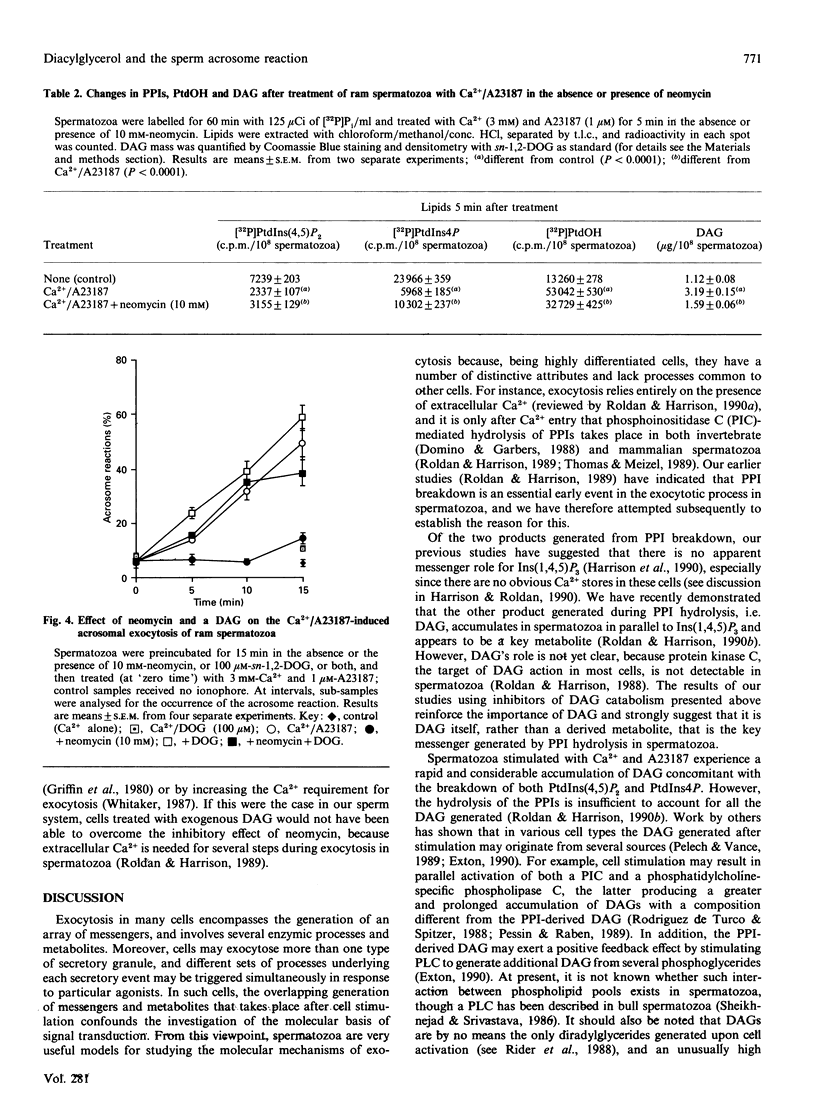
When ram spermatozoa were treated with Ca2+ and the ionophore A23187 to induce acrosomal exocytosis, a rise in diacylglycerol (DAG) mass was observed, concomitant with a rapid breakdown of [32P]P1-labelled phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 4-phosphate and a rise in [32P]Pi-labelled phosphatidate. Inclusion of the DAG lipase inhibitor RHC 80267 resulted in further but biphasic increases in DAG; there was an increasing accumulation of DAG with concentrations of RHC 80267 up to 10 microM, whereas higher concentrations produced lessening accumulation. Inclusion of RHC 80267 in the ionophore induction system also resulted in significant accelerations of the onset of exocytosis. In spermatozoa stimulated with Ca2+/A23187 and the DAG kinase inhibitor R59022, a similar increase in DAG levels together with stimulation of acrosomal exocytosis were observed. Preincubation of spermatozoa with sn-1-oleoyl-2-acetylglycerol, rac-1-oleoyl-2-acetylglycerol, sn-1,2-dioctanoylglycerol and sn-1,3-dioctanoylglycerol before treatment with Ca2+/A23187 resulted in a dose-dependent stimulation of exocytosis by all these isomers. Neomycin inhibited Ca2+/A23187-induced generation of DAG together with polyphosphoinositide breakdown, as well as acrosomal exocytosis. Inclusion of exogenous DAG, however, overcame the inhibitory effect of neomycin on exocytosis. Our results suggest that DAG has a key role in acrosomal exocytosis and that it acts as a messenger rather than as a substrate from which other active metabolites are generated. The lack of stereospecificity shown by the exogenous DAGs implies that DAG does not act by stimulating protein kinase C, but the metabolite's actual target in the sperm cell is as yet unclear.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bauldry S. A., Wykle R. L., Bass D. A. Phospholipase A2 activation in human neutrophils. Differential actions of diacylglycerols and alkylacylglycerols in priming cells for stimulation by N-formyl-Met-Leu-Phe. J Biol Chem. 1988 Nov 15;263(32):16787–16795. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Assembly of phospholipids into cellular membranes: biosynthesis, transmembrane movement and intracellular translocation. Annu Rev Cell Biol. 1988;4:579–610. doi: 10.1146/annurev.cb.04.110188.003051. [DOI] [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Wilson P. B., Exton J. H. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987 Nov 5;262(31):15309–15315. [PubMed] [Google Scholar]
- Boni L. T., Rando R. R. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J Biol Chem. 1985 Sep 5;260(19):10819–10825. [PubMed] [Google Scholar]
- Carney D. H., Scott D. L., Gordon E. A., LaBelle E. F. Phosphoinositides in mitogenesis: neomycin inhibits thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. Cell. 1985 Sep;42(2):479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Chang J. P., Morgan R. O., Catt K. J. Dependence of secretory responses to gonadotropin-releasing hormone on diacylglycerol metabolism. Studies with a diacylglycerol lipase inhibitor, RHC 80267. J Biol Chem. 1988 Dec 15;263(35):18614–18620. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Diacylglycerol causes major structural transitions in phospholipid bilayer membranes. Biochem Biophys Res Commun. 1984 Oct 30;124(2):491–496. doi: 10.1016/0006-291x(84)91580-8. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Bray J., Quinn P. J. Long-chain unsaturated diacylglycerols cause a perturbation in the structure of phospholipid bilayers rendering them susceptible to phospholipase attack. Biochem Biophys Res Commun. 1984 Dec 14;125(2):836–842. doi: 10.1016/0006-291x(84)90615-6. [DOI] [PubMed] [Google Scholar]
- Domino S. E., Garbers D. L. The fucose-sulfate glycoconjugate that induces an acrosome reaction in spermatozoa stimulates inositol 1,4,5-trisphosphate accumulation. J Biol Chem. 1988 Jan 15;263(2):690–695. [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Fleming A. D., Yanagimachi R. Evidence suggesting the importance of fatty acids and the fatty acid moieties of sperm membrane phospholipids in the acrosome reaction of guinea pig spermatozoa. J Exp Zool. 1984 Mar;229(3):485–489. doi: 10.1002/jez.1402290317. [DOI] [PubMed] [Google Scholar]
- Griffin H. D., Sykes M., Hawthorne J. N. Effects of neomycin on calcium and polyphosphoinositide metabolism of guinea pig synaptosomes. J Neurochem. 1980 Mar;34(3):750–752. doi: 10.1111/j.1471-4159.1980.tb11209.x. [DOI] [PubMed] [Google Scholar]
- Harrison R. A., Dott H. M., Foster G. C. Bovine serum albumin, sperm motility, and the "dilution effect'. J Exp Zool. 1982 Jul 20;222(1):81–88. doi: 10.1002/jez.1402220111. [DOI] [PubMed] [Google Scholar]
- Harrison R. A., Roldan E. R., Lander D. J., Irvine R. F. Ram spermatozoa produce inositol 1,4,5-trisphosphate but not inositol 1,3,4,5-tetrakisphosphate during the Ca2+/ionophore-induced acrosome reaction. Cell Signal. 1990;2(3):277–284. doi: 10.1016/0898-6568(90)90055-f. [DOI] [PubMed] [Google Scholar]
- Harrison R. A., Roldan E. R. Phosphoinositides and their products in the mammalian sperm acrosome reaction. J Reprod Fertil Suppl. 1990;42:51–67. [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Stimulation of phosphatidylinositol 4,5-bisphosphate phospholipase C activity by phosphatidic acid. Arch Biochem Biophys. 1989 Feb 1;268(2):516–524. doi: 10.1016/0003-9861(89)90318-4. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Checani G. C., Deykin D. Stimulation of Ca2+-activated human platelet phospholipase A2 by diacylglycerol. Biochem J. 1987 Dec 15;248(3):779–783. doi: 10.1042/bj2480779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre R. N., King W. C., MacDonald M. L., Glomset J. A. Distribution of distinct arachidonoyl-specific and non-specific isoenzymes of diacylglycerol kinase in baboon (Papio cynocephalus) tissues. Biochem J. 1990 Feb 15;266(1):291–299. doi: 10.1042/bj2660291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meizel S., Turner K. O. Stimulation of an exocytotic event, the hamster sperm acrosome reaction, by cis-unsaturated fatty acids. FEBS Lett. 1983 Sep 19;161(2):315–318. doi: 10.1016/0014-5793(83)81032-1. [DOI] [PubMed] [Google Scholar]
- Mitchell K. T., Ferrell J. E., Jr, Huestis W. H. Separation of phosphoinositides and other phospholipids by two-dimensional thin-layer chromatography. Anal Biochem. 1986 Nov 1;158(2):447–453. doi: 10.1016/0003-2697(86)90574-9. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Handa S. Coomassie brilliant blue staining of lipids on thin-layer plates. Anal Biochem. 1984 Nov 1;142(2):406–410. doi: 10.1016/0003-2697(84)90484-6. [DOI] [PubMed] [Google Scholar]
- Neill A. R., Masters C. J. Metabolism of fatty acids by ovine spermatozoa. J Reprod Fertil. 1973 Aug;34(2):279–287. doi: 10.1530/jrf.0.0340279. [DOI] [PubMed] [Google Scholar]
- Nikolopoulou M., Soucek D. A., Vary J. C. Changes in the lipid content of boar sperm plasma membranes during epididymal maturation. Biochim Biophys Acta. 1985 May 28;815(3):486–498. doi: 10.1016/0005-2736(85)90377-3. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nomura H., Ase K., Sekiguchi K., Kikkawa U., Nishizuka Y., Nakano Y., Satoh T. Stereospecificity of diacylglycerol for stimulus-response coupling in platelets. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1143–1151. doi: 10.1016/0006-291x(86)90754-0. [DOI] [PubMed] [Google Scholar]
- Pessin M. S., Raben D. M. Molecular species analysis of 1,2-diglycerides stimulated by alpha-thrombin in cultured fibroblasts. J Biol Chem. 1989 May 25;264(15):8729–8738. [PubMed] [Google Scholar]
- Rando R. R., Young N. The stereospecific activation of protein kinase C. Biochem Biophys Res Commun. 1984 Jul 31;122(2):818–823. doi: 10.1016/s0006-291x(84)80107-2. [DOI] [PubMed] [Google Scholar]
- Rider L. G., Dougherty R. W., Niedel J. E. Phorbol diesters and dioctanoylglycerol stimulate accumulation of both diacylglycerols and alkylacylglycerols in human neutrophils. J Immunol. 1988 Jan 1;140(1):200–207. [PubMed] [Google Scholar]
- Rodriguez de Turco E. B., Spitzer J. A. Kinetics of diacylglycerol accumulation in response to vasopressin stimulation in hepatocytes of continuously endotoxaemic rats. Biochem J. 1988 Jul 1;253(1):73–79. doi: 10.1042/bj2530073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan E. R., Harrison R. A. Absence of active protein kinase C in ram spermatozoa. Biochem Biophys Res Commun. 1988 Sep 15;155(2):901–906. doi: 10.1016/s0006-291x(88)80581-3. [DOI] [PubMed] [Google Scholar]
- Roldan E. R., Harrison R. A. Diacylglycerol and phosphatidate production and the exocytosis of the sperm acrosome. Biochem Biophys Res Commun. 1990 Oct 15;172(1):8–15. doi: 10.1016/s0006-291x(05)80165-2. [DOI] [PubMed] [Google Scholar]
- Roldan E. R., Harrison R. A. Polyphosphoinositide breakdown and subsequent exocytosis in the Ca2+/ionophore-induced acrosome reaction of mammalian spermatozoa. Biochem J. 1989 Apr 15;259(2):397–406. doi: 10.1042/bj2590397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Selivonchick D. P., Schmid P. C., Natarajan V., Schmid H. H. Structure and metabolism of phospholipids in bovine epididymal spermatozoa. Biochim Biophys Acta. 1980 May 28;618(2):242–254. doi: 10.1016/0005-2760(80)90030-2. [DOI] [PubMed] [Google Scholar]
- Sheikhnejad R. G., Srivastava P. N. Isolation and properties of a phosphatidylcholine-specific phospholipase C from bull seminal plasma. J Biol Chem. 1986 Jun 5;261(16):7544–7549. [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Sundler R., Papahadjopoulos D. Control of membrane fusion by phospholipid head groups. I. Phosphatidate/phosphatidylinositol specificity. Biochim Biophys Acta. 1981 Dec 21;649(3):743–750. doi: 10.1016/0005-2736(81)90179-6. [DOI] [PubMed] [Google Scholar]
- Sutherland C. A., Amin D. Relative activities of rat and dog platelet phospholipase A2 and diglyceride lipase. Selective inhibition of diglyceride lipase by RHC 80267. J Biol Chem. 1982 Dec 10;257(23):14006–14010. [PubMed] [Google Scholar]
- Thomas P., Meizel S. Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochem J. 1989 Dec 1;264(2):539–546. doi: 10.1042/bj2640539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M., Aitchison M. Calcium-dependent polyphosphoinositide hydrolysis is associated with exocytosis in vitro. FEBS Lett. 1985 Mar 11;182(1):119–124. doi: 10.1016/0014-5793(85)81167-4. [DOI] [PubMed] [Google Scholar]
- Whitaker M. How calcium may cause exocytosis in sea urchin eggs. Biosci Rep. 1987 May;7(5):383–397. doi: 10.1007/BF01362502. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D. C., Roevens P., Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem. 1985 Dec 15;260(29):15762–15770. [PubMed] [Google Scholar]


