Abstract
Background
Patients with type 2 diabetes mellitus (DM) comprise more than a quarter of all patients undergoing percutaneous coronary intervention and are at higher risk of adverse events. We sought to reexamine the optimal duration of dual antiplatelet therapy (DAPT) postpercutaneous coronary intervention in patients with DM.
Methods
We systematically included randomized controlled trials comparing any 2 of 1, 3, 6, and 12 months of DAPT that reported major adverse cardiovascular events (MACE), net adverse clinical events (NACE), bleeding, or stent thrombosis in DM, and performed a frequentist network meta-analysis. We also performed a sensitivity analysis of trials that exclusively enrolled patients with acute coronary syndrome.
Results
In 16 randomized controlled trials comprising 16,376 adults with DM, there was no significant difference in NACE, MACE, stent thrombosis, or major bleeding between pairwise comparisons of 1, 3, 6, and 12 months of DAPT, except for a signal for lower bleeding with 3 months of DAPT compared to 12 (risk ratio, 0.72; 95% CI, 0.51-0.99). Sensitivity analysis of trials that solely included acute coronary syndrome similarly showed no significant difference in MACE between 1, 3, 6, and 12 months of DAPT.
Conclusions
Our study found no meaningful difference in NACE or MACE between pairwise comparisons of 1, 3, 6, and 12 months of DAPT by study-level meta-analysis of patients with DM, with lower bleeding risk observed with 3 months than with 12 months of DAPT. This finding may provide clinicians greater flexibility to personalize patients’ DAPT duration based on other non-DM comorbidities that might affect bleeding or thrombosis risk.
Keywords: diabetes mellitus, dual antiplatelet therapy, duration, meta-analysis, percutaneous coronary intervention
Introduction
Type 2 diabetes mellitus (DM) affects approximately 462 million people globally,1 among which a third have comorbid atherosclerotic cardiovascular disease (ASCVD).2 Patients with DM comprise more than a quarter of all patients undergoing percutaneous coronary intervention (PCI) and are at higher risk of adverse clinical outcomes such as cardiac death, myocardial infarction, in-stent restenosis, and need for repeat revascularization.3, 4, 5 DM poses a special challenge in ASCVD as it is associated with more complex coronary lesions that are multivessel and diffusely distributed.6,7 Moreover, DM is characterized by a state of increased platelet reactivity and abnormal platelet function.8
Given the rapid expansion of the global DM burden coupled with greater ischemic risks, increased platelet reactivity, and higher complexity coronary lesions in patients with DM, determining optimal dual antiplatelet therapy (DAPT) duration in this population is essential.9 The 2021 guidelines from the American College of Cardiology, American Heart Association, and Society for Cardiovascular Angiography and Interventions, and the 2023 guidelines from the European Society of Cardiology recommend 6 months of DAPT after PCI for stable ischemic heart disease and 12 months of DAPT after PCI for acute coronary syndrome (ACS) regardless of the presence of DM, but with the allowance of abbreviated DAPT tailored to the needs of the patient.10,11 However, whether this should be tailored in patients with DM remains uncertain. Thus, we set out to determine whether there are clinically important differences in ischemic and bleeding outcomes with different durations of DAPT in patients with DM using data from randomized controlled trials (RCTs).
Methods
We conducted our systematic review following a documented protocol found on Open Science Framework12 and followed the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplemental Table S1).13 This study was exempt from institutional review board approval as it exclusively used data from previously published sources.
Search strategy and inclusion criteria
The co-first authors carried out a thorough literature search across multiple databases, which included the Cochrane Library, Ovid Embase, Ovid MEDLINE, PubMed, Scopus, and Web of Science Core Collection. We also searched for pertinent trials in the proceedings of major international cardiology conferences, which included the American College of Cardiology, American Heart Association, European Society of Cardiology, and Society for Cardiovascular Angiography & Interventions. This search was constructed using carefully selected combinations of controlled and free text terms related to topics such as DAPT, treatment duration, PCI, and RCT, mirroring the approach taken in a prior study.14 The search period included articles from the inception of these databases up until August 12, 2023. Only articles in English were taken. The complete search strategies for all databases can be found in Supplemental Table S2. Upon compiling pertinent studies, the reference lists of each study were cross-referenced to identify additional relevant literature. To eliminate duplicate studies, citations from the initial search were imported into EndNote 20 software and screened using Covidence. Two independent reviewers (D.P. and J.H.) performed title, abstract, and full-text review with disagreement resolved by the corresponding author (M.N.). Any discrepancies were resolved through team discussions under the corresponding author’s supervision.
Studies with the following criteria were included: (1) RCT; (2) comparison of any 2 of 1, 3, 6, or 12 months of DAPT; (3) reporting of outcomes associated with patients with diabetes mellitus; (4) follow-up duration ≥9 months from the index PCI; (5) written in English language. When ≥2 studies on the same RCT data were found, the earlier original paper was prioritized. For each selected trial, we utilized the Cochrane Collaboration’s tool to evaluate the risk of bias, and for each pooled outcome, we used the GRADE system to assess its quality.15,16
Data acquisition and outcomes of interest
From each individual study, we gathered specific details including year of enrollment, the countries where the study took place, study sample size, the proportion of patients with ACS, the specific single antiplatelet agent used, and the types of stents deployed. Additionally, we compiled baseline patient characteristics to facilitate study-level comparisons. We did not have access to patient-level data for the studies included in the present analysis. All included variables were abstracted from published materials. The primary outcome was net adverse clinical events (NACE). Secondary outcomes included major adverse cardiovascular events (MACE), bleeding, and definite or probable stent thrombosis (ST).
Statistical analysis
To ensure uniformity across all studies, risk ratios were manually computed from the selected RCTs. Modified Haldane-Anscombe correction was used to resolve 0-cell problems.17 Following the outcome compilation, a frequentist network meta-analysis with random effects model was performed to determine pooled estimates by alternating the reference groups. Inconsistencies between direct and indirect estimates were evaluated through node-splitting analysis. Heterogeneity in the network models was assessed using Tau-squared and I-squared values. For each DAPT duration, P-scores were computed for each outcome. These scores were considered meaningful only when the network meta-analysis indicated significant distinctions among various DAPT durations. P-scores indicate the average level of certainty that a particular DAPT duration is superior to other durations, weighted equally across all denominators.18 P-scores do not have a universal threshold of significance but serve to rank the treatments in direct and indirect comparisons, with a P-score of 0 representing the treatment with the lowest effectiveness and safety within the network, and a score of 1 representing the treatment with the highest effectiveness and safety within the network. In node-splitting analysis, a 2-tailed P value <.05 was considered statistically significant. A sensitivity analysis including trials that reported major bleeding, as defined by Bleeding Academic Research Consortium type 3 to 5 bleeding or thrombolysis in myocardial infarction major bleeding, was performed. Another sensitivity analysis excluding trial(s) outcomes presented in abstract only in diabetic subgroups was also conducted. In addition, a sensitivity analysis including trials that included only patients with ACS was performed for the outcomes NACE and MACE. Pooled outcomes for bleeding and ST in ACS could not be generated because of the lack of relevant trials that reported these outcomes in patients with DM. Frequent network meta-analysis was performed using meta and netmeta packages in R version 4.2.3 (R Foundation for Statistical Computing).
Results
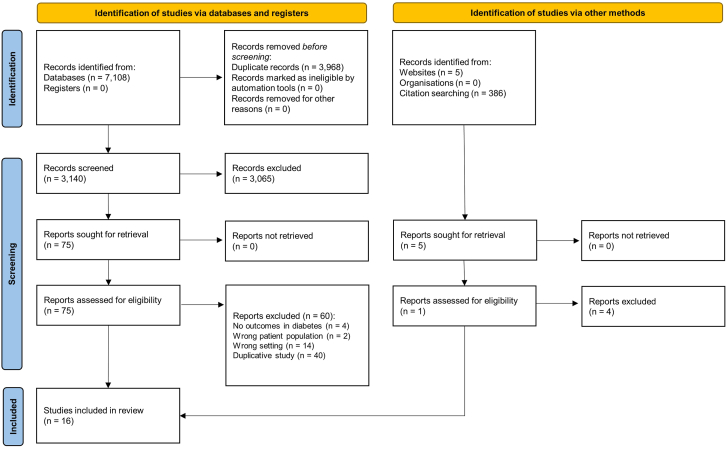
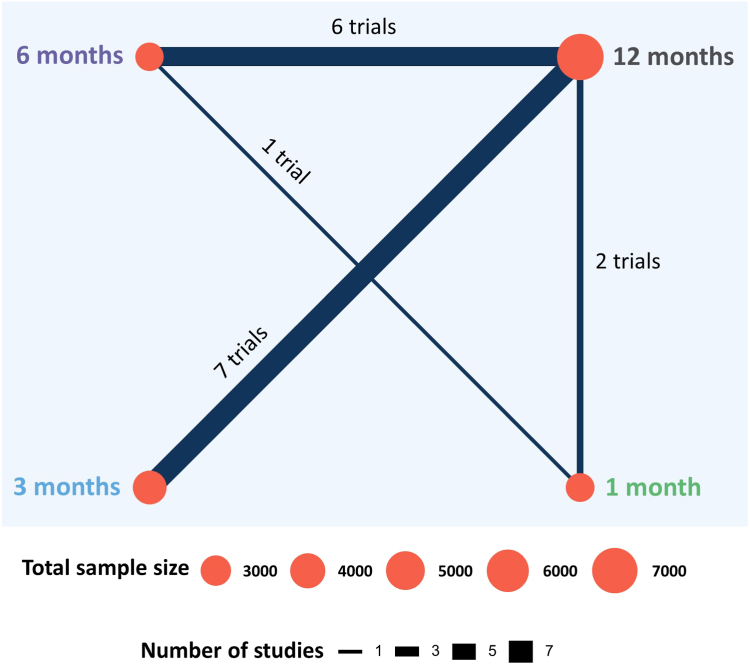
Sixteen RCTs with a cumulative sample size of 16,376 patients with DM were included in our study (Figure 1).19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 All the data were collected from full manuscripts except for 1 trial whose outcomes in patients with DM were presented in a conference abstract.35 Seven trials, which included 7365 (45.0%) patients with DM, compared 3 months with 12 months of DAPT (Figure 2).19,22,23,26, 27, 28,33 Six trials, which included 3621 (22.1%) patients with DM, compared 6 months with 12 months of DAPT.21,24,25,29,32,34 Two trials, which included 3852 (23.5%) patients with DM, compared 1 month with 12 months of DAPT.20,31 One trial, which included 1538 (9.4%) patients with DM, compared 1 month with 6 months of DAPT.30 Years of recruitment ranged from 2008 to 2021 (Table 1).19, 21, 22, 23, 24, 26, 27, 28, 29, 30, 32, 34, 35, 36, 37, 38 The proportion of ACS cases within the selected trials varied between 32% and 100%, with an unadjusted mean of 60%. Four trials, including the Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent 2 (STOPDAPT-2) ACS trial, which was the continuation of the STOPDAPT-2 trial, exclusively enrolled patients with ACS.19,26,34,36 The percentage of patients with DM in the trials ranged from 21% to 39%. Aspirin was the exclusive single antiplatelet agent after DAPT discontinuation across 9 trials, followed by 3 trials that prescribed ticagrelor and 2 trials that prescribed clopidogrel. One trial employed both aspirin and clopidogrel, while another allowed physicians to decide, with a prevailing preference for aspirin (64.1%), followed by clopidogrel (33.7%). There was notable diversity in terms of baseline and procedural characteristics among the trials (Supplemental Table S3). However, both patients with and without DM are included in this table as baseline and procedural characteristics by diabetes status were not available in many of the trials. The definitions of NACE, MACE, and bleeding also differed from one trial to another (Supplemental Table S4). ST, however, was defined according to Academic Research Consortium in all the trials.39
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the meta-analysis. The flow diagram illustrates the process whereby studies were selected in the present network meta-analysis.
Figure 2.
Network plot of the included randomized controlled trials. The network plot illustrates the number of trials and patients with diabetes mellitus among trials that compared 1 month, 3 months, 6 months, and 12 months of dual antiplatelet therapy. The size of the blue circles and blue lines are proportional to the total sample size of patients with diabetes mellitus and the number of relevant trials, respectively.
Table 1.
Main characteristics of the included trials.
| Trial | Enrollment years | Country | ACSa | DMb | SAPT | Stent | Experimental |
Control |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Sized | DAPT | Sized | DAPT | |||||||
| HOST-IDEA23 | 2016-2021 | South Korea | 55.2% | 39% | Anyc | Biodegradable or polymer-free SES | 406 | 3 mo | 378 | 12 mo |
| MASTER DAPT30 | 2017-2019 | Multinational | 48.3% | 34% | Clopidogrel, aspirin | SES | 754 | 1 mo | 784 | 6 mo |
| TICO26 | 2015-2018 | South Korea | 100% | 27% | Ticagrelor | SES | 418 | 3 mo | 417 | 12 mo |
| SMART-CHOICE22 | 2014-2017 | South Korea | 58.2% | 38% | Clopidogrel | EES, SES | 923 | 3 mo | 899 | 12 mo |
| TWILIGHT28 | 2015-2017 | Multinational | 64.8% | 37% | Ticagrelor | Second-generation DESe | 570 | 3 mo | 552 | 12 mo |
| STOPDAPT-236 | 2015-2017 | Japan | 38.2% | 39% | Clopidogrel | Cobalt-chromium EES | 1303 | 1 mo | 1317 | 12 mo |
| REDUCE19 | 2014-2016 | Multinational | 100% | 21% | Aspirin | CD34+ antibody-coated SES | 1018 | 3 mo | 1012 | 12 mo |
| GLOBAL LEADERS37 | 2013-2015 | Multinational | 50.6% | 24% | Ticagrelor | BES | 162 | 1 mo | 145 | 12 mo |
| SMART-DATE34 | 2012-2015 | South Korea | 100% | 28% | Aspirin | ZES, EES, BES | 378 | 6 mo | 365 | 12 mo |
| IVUS-XPL38 | 2010-2014 | South Korea | 49.0% | 37% | Aspirin | EES | 211 | 6 mo | 203 | 12 mo |
| SECURITY32 | 2009-2014 | Multinational | 38.4% | 31% | Aspirin | ZES, BES, EES | 249 | 6 mo | 257 | 12 mo |
| ISAR-SAFE29 | 2008-2014 | Multinational | 40.7% | 25% | Aspirin | EES, SES, ZES, BES, PES | 206 | 6 mo | 223 | 12 mo |
| I-LOVE-IT 224 | 2012-2013 | China | 81.8% | 23% | Aspirin | Biodegradable-polymer SES | 495 | 6 mo | 484 | 12 mo |
| OPTIMIZE35 | 2010-2012 | Brazil | 32.0% | 34% | Aspirin | ZES | 554 | 3 mo | 549 | 12 mo |
| RESET27 | 2009-2010 | South Korea | 54.6% | 29% | Aspirin | ZES | 316 | 3 mo | 305 | 12 mo |
| EXCELLENT21 | 2008-2009 | South Korea | 48.5% | 38% | Aspirin | EES, SES | 269 | 6 mo | 281 | 12 mo |
ACS, acute coronary syndrome; BES, biolimus-eluting stent; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; DM, diabetes mellitus; EES, everolimus-eluting stent; PES, paclitaxel-eluting stent; SAPT, single antiplatelet therapy; SES, sirolimus-eluting stent; ZES, zotarolimus-eluting stent.
Average of the percentage of acute coronary syndrome in abbreviated and standard dual antiplatelet groups.
Percentage of patients with diabetes in the total sample of the original trial.
Any antiplatelet at the discretion of the ordering physician: aspirin (64.1%), clopidogrel (33.7%), ticagrelor (1.9%), prasugrel (0.3%) in the trial.
Sample size of the population with diabetes mellitus.
Second-generation drug-eluting stent: durable polymer cobalt-chromium EES, durable polymer platinum-chromium EES, durable polymer ZES, durable polymer cobalt-chromium SES, biodegradable polymer DES, polymer-free DES, bioresorbable vascular scaffold, sirolimus-eluting self-apposing stent, tacrolimus-eluting carbostent.
The risk of bias was mostly low in the selected trials, except for performance bias which was high in 10 trials due to the open-label design (Supplemental Table S5). Quality of pooled outcomes was moderate owing to some biases and imprecisions (Supplemental Table S6). Heterogeneity observed in the frequent network models ranged from none to moderate (Supplemental Table S7). No inconsistencies in the frequentist network models were observed with random effects applied. The results of the node-splitting analysis of inconsistency can be found in the supplementary material (Supplemental Table S8 and Supplemental Figure S1).
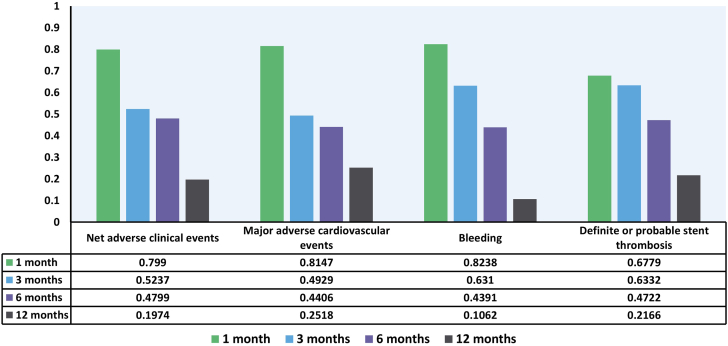
Although numerically lower, no significant difference in the risk of NACE was observed between 1 month and 12 months of DAPT, between 3 months and 12 months of DAPT, and between 6 months and 12 months of DAPT (Table 2) in patients with DM. Similarly, no difference in the risk of NACE was seen between 1 and 6 months of DAPT and between 3 and 6 months of DAPT. There was also no difference in the risk of NACE between 1 and 3 months of DAPT. For NACE, P-score was highest in 1 month of DAPT, followed by 3, 6, and 12 months of DAPT (Figure 3).
Table 2.
Pooled estimates of frequentist network meta-analysis for each outcome.
 |
The duration of dual antiplatelet therapy at the rightmost column serves as the reference group for the respective column.
Figure 3.
P-scores of each duration of dual antiplatelet therapy. The bar graphs demonstrate the P-scores of 1 month (green), 3 months (blue), 6 months (purple), and 12 months (gray) of dual antiplatelet therapy after percutaneous coronary intervention in patients with diabetes. P-scores measure the extent of certainty that the specified duration of dual antiplatelet therapy is better than other durations of dual antiplatelet therapy.
The risk of MACE was not different between all the combinations of 1, 3, 6, and 12 months of DAPT. Three months of DAPT was associated with lower bleeding (risk ratio, 0.72; 95% CI, 0.51-0.99) compared with 12 months of DAPT. However, there was no significant difference in the risk of bleeding between 1 and 3 months of DAPT, 1 and 6 months of DAPT, and 3 and 6 months of DAPT. No difference in the risk of ST was observed between all the combinations of 1, 3, 6, and 12 months of DAPT. P-score was highest in 1 month of DAPT, followed by 3, 6, and 12 months of DAPT for each of the outcomes, MACE, bleeding, and ST. The event rates of NACE, MACE, bleeding, and ST can be found in Supplemental Table S9.
Sensitivity analysis including trials that reported major bleeding demonstrated similar results, with 3 months of DAPT therapy associated with lower risk of major bleeding compared with 12 months of DAPT (Supplemental Table S10). Sensitivity analysis that excluded the Optimized Duration of Clopidogrel Therapy Following Treatment With the Zotarolimus-Eluting Stent in Real-World Clinical Practice (OPTIMIZE) trial, which only reported diabetic patient outcomes in abstract not manuscript,35 showed similar results (Supplemental Table S11). Sensitivity analysis including trials that exclusively enrolled patients with ACS showed no difference in NACE or MACE between all the combinations of 1, 3, 6, and 12 months of DAPT (Supplemental Table S12).
Discussion
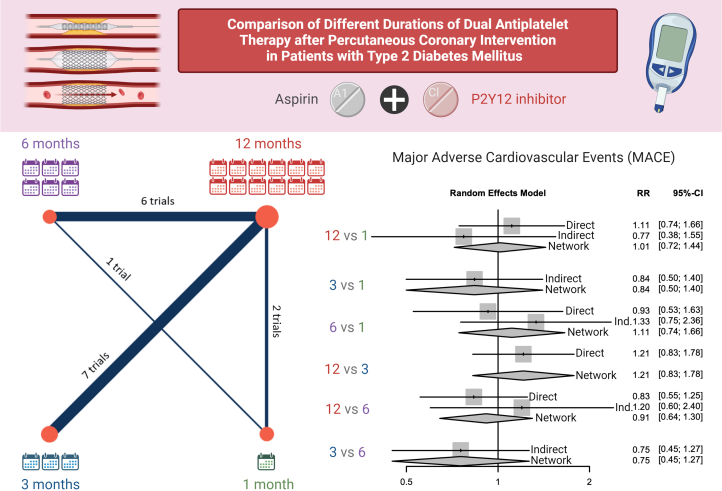
In this network meta-analysis of 16 RCTs of patients with DM undergoing PCI, we found no significant difference in NACE or MACE between pairwise comparisons of 1, 3, 6, and 12 months of DAPT (Central Illustration). In light of recent meta-analyses comparing DAPT durations ranging from 12 to 48 months in patients with DM,40, 41, 42, 43 we focused our investigation on outcomes associated with DAPT durations of 1, 3, 6, and 12 months. Although 3 months of DAPT was associated with lower risk of bleeding compared with 12 months of DAPT, there was no significant difference in the risk of bleeding between 1 and 3 months, 1 and 6 months, and 3 and 6 months of DAPT. Notably, we found that there was no increase in the risk of ST with shorter durations of DAPT, although comparisons across DAPT durations were limited by the low number of ST events. These findings have important implications in the otherwise highly complex population of patients with DM undergoing PCI, as they suggest that there is no significant benefit to longer-term (6-12 months) DAPT and no corresponding rise in-stent thrombosis risk to shorter-term (1-3 months) DAPT at a population level. This allows clinicians greater flexibility to personalize patients’ DAPT duration based on other non-DM comorbidities that might affect bleeding or thrombosis risk.
Central Illustration.
Comparison of 1 (in green), 3 (in blue), 6 (in purple), and 12 (in red) months duration of dual antiplatelet therapy after percutaneous coronary intervention in patients with diabetes mellitus. On left, the size of the circles and thickness of the paths between nodes reflect the number of randomized controlled trials involved in the comparison. On right, the relative risk (RR) of major adverse cardiovascular events (MACE) is shown with 95% CI for direct comparison of effect, indirect comparison of effect, and network comparison of effect in this network meta-analysis.
Patients with DM are a special population at higher risk of ASCVD coupled with increased platelet reactivity, platelet aggregation, and risk of thrombosis.44 In patients with coronary artery disease, platelet aggregation is higher in those with DM compared with those without DM, and among those with DM, the effect is most pronounced for those requiring insulin therapy.45 The mechanisms of this increased risk in DM are well-elucidated at the molecular level. Hyperglycemia suppresses the expression of microRNA (miR-223, miR-26b, miR-126, miR-140), causing an upregulation of P2RY12 and SELP target mRNA, causing increased expression of P2Y12 receptors and P-selectin on the platelet surface of patients with diabetes.46 Patients with DM have higher levels of coagulation factors II, V, VII, VIII, and X and lower levels of anticoagulant protein C compared with subjects without diabetes.47 Because of the increased platelet reactivity and thrombosis risk in patients with DM, there has been concern about whether patients with DM require a more aggressive DAPT strategy post-PCI.48,49 In fact, the presence of DM is a consideration for prolonging DAPT beyond the initial 1 year according to the widely-used DAPT score.49 This contrasts with the present findings suggesting similar ischemic outcomes with abbreviated DAPT without a concomitant increase in risk of bleeding.
Prior meta-analyses of DAPT duration in patients with DM by Gargiulo et al,40 Sharma et al,42 and Zhang et al41 have not consistently shown benefit to prolonged (12-48 months) DAPT duration. In at least 1 meta-analysis, prolonged DAPT was associated with increased risk of major or minor bleeding, an effect that was seen in both patients with or without diabetes.40 More recently, in 2021, An et al43 conducted a meta-analysis of 18 RCTs of patients with DM, investigating the effect of short-term DAPT (defined as 1-3 months), medium-term DAPT (defined as 6 months), standard-term DAPT (defined as 12 months), and extended-term DAPT (defined as 24-48 months) on all-cause mortality, cardiac mortality, myocardial infarction, stroke, target vessel revascularization, probable ST, or major bleeding. Of note, the primary end points in the meta-analysis were the same as in the individual trials, resulting in an unusually high level of heterogeneity. In contrast with prior studies,41,42 they performed a Bayesian network analysis, allowing for estimation of treatment effects between interventions that have not been directly compared. An et al43 found that short-term (1-3 months) DAPT and standard-term (12 months) DAPT were associated with a reduction in the primary end point as individually defined in each trial, compared with extended-term (24-48 months) DAPT. Importantly, however, there was no difference in all-cause mortality, cardiac mortality, myocardial infarction, stroke, target vessel revascularization, definite or ST, and major bleeding across short-term, medium-term, standard-term, or extended-term DAPT. The meta-analyses convincingly demonstrated no benefit (and potential harm) associated with prolonging DAPT beyond 12 months in patients with DM.40, 41, 42 Given the findings of these recent studies,40, 41, 42, 43 we focused the scope of the present meta-analysis a priori on DAPT durations of 1, 3, 6, and 12 months—representing the only meta-analysis (and the only network meta-analysis) of these durations in patients with DM to our knowledge. The network geometry of our present analysis additionally differs from that of An et al43 because they treated 1 month and 3 months as the same node (“≤3 months”); 24, 30, 36, and 48 months were also treated as the same node (“>12 months”). We chose to exclude studies involving a comparator beyond 12 months. In addition, since the time of the most recently published meta-analysis on this subject, the HOST-IDEA trial was subsequently completed and published. The MASTER DAPT and TICO trials were published near the time of the prior meta-analysis but not included in that study. Our present meta-analysis adds important data from these interim trials to provide updated pooled estimates of effect size. While the results of the STOPDAPT-3 trial were presented as a late-breaking trial at the ESC 2023, specific results of the subgroup of patients with DM are not yet available for inclusion in meta-analysis.
Of note, this study focused on determining the optimal duration of DAPT. However, the choice of antiplatelet agent is also an important consideration, as not all P2Y12 inhibitors are metabolized similarly. Clopidogrel, the most commonly used P2Y12 inhibitor, induces a lower amount of platelet inhibition in patients with DM compared to those without DM.50 Active metabolites of clopidogrel are lower in patients with DM than those without DM after a 600 mg load.51 In contrast, active metabolites of prasugrel do not exhibit different levels in patients with or without DM,52 and ticagrelor is a direct-acting agent that binds noncompetitively to the P2Y12 receptor without the need for transformation into an active metabolite.53 Given this, in the TRITON-TIMI 38 trial, use of prasugrel-based DAPT was associated with a greater reduction in ischemic events compared with clopidogrel-based DAPT in patients with DM undergoing PCI for ACS, without an increase in major bleeding.54 In PLATO, ticagrelor-based DAPT was associated with a greater reduction in ischemic events compared with clopidogrel-based DAPT, although this finding was true in both patients with and without DM.55 Therefore, preferential use of ticagrelor or prasugrel over clopidogrel may be one way to tailor DAPT for patients with DM. Overall, the differential metabolization of P2Y12 inhibitors in patients with DM underscores the importance of treating patients with DM as a distinct population when considering the choice and duration of DAPT after PCI. Notably, these findings were made in RCTs that were not designed or powered to assess the efficacy of P2Y12 inhibitor choice in the DM subgroup alone.
This meta-analysis should be considered in the context of several limitations. Data on patients with DM derive from post hoc or prespecified subgroup analyses of RCTs, which is a limitation that carries over from the initial trials to any meta-analysis of these trials, including our meta-analysis. Because we did not have access to patient-level data, it was not possible to perform subgroup analysis by clinical presentation in the subset of patients with diabetes nor was it possible to create a unified composite outcome for MACE or NACE, whose definitions were different in each trial. For example, ST was included in the definition of MACE and NACE for TICO, the definition of NACE for STOPDAPT-2, HOST-IDEA, REDUCE, ISAR-SAFE, RESET, but not in the definitions of MACE or NACE in the other trials. Therefore, it is not possible for us to make a statement on whether there are benefits or disadvantages to a particular duration of DAPT with respect to risk of ST. Similarly, it was not possible to adjust for confounding factors such as the use of ancillary imaging, patient and procedural characteristics, stent types, and medications, which should be the focus of future RCTs. In addition, our study utilized data from existing RCTs and was limited by the sample size available from patients enrolled in those trials. Thus, the present analysis may not be powered to detect small but clinically relevant differences between DAPT durations to provide definitive conclusions, especially for rarer outcome events such as ST. Many of the trials were conducted as open-label trials, giving rise to possible performance bias. Moreover, the choice of post-DAPT single antiplatelet therapy remains a source of heterogeneity across trials. Finally, diabetes was not classified into type 1 or 2, and patients with prolonged DM and potentially more severe coronary artery disease may have been excluded from the trials, so our results may not be generalizable to the entire patient population with DM.
In conclusion, in this network meta-analysis of 16 RCTs of patients with DM undergoing PCI, we found no meaningful difference in NACE or MACE between pairwise comparisons of 1, 3, 6, and 12 months of DAPT, with a lower bleeding risk associated with 3 months compared to 12 months of DAPT. The suggestion that there is no significant benefit to longer-term DAPT and no corresponding rise in MACE risk to shorter-term DAPT at a population level, while a “negative” result, is an important one as it provides clinicians greater freedom to personalize patients’ DAPT duration based on other non-DM comorbidities that might affect bleeding or thrombosis risk.
Acknowledgments
Declaration of competing interest
Michael G. Nanna reports current research support from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation and the Patient-Centered Outcomes Research Institute (PCORI), and consulting from HeartFlow and Merck. Dae Yong Park, Jiun-Ruey Hu, Greta Campbell, Kiara Goldwag, Michelle D. Kelsey, S. Elissa Altin, and Cesia Gallegos-Kattán report no financial interests.
Funding sources
Michael G. Nanna reports research funding from the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342) and the National Institute on Aging/National Institutes of Health from R03AG074067 (GEMSSTAR award).
Ethics statement and patient consent
The authors retrieved and synthesized data from previously published studies; therefore, no ethical approval was required or obtained.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2024.101859.
Supplementary material
References
- 1.Khan M.A.B., Hashim M.J., King J.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einarson T.R., Acs A., Ludwig C., Panton U.H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J., Han J.K., Chang M., et al. Impact of intensive glucose control in patients with diabetes mellitus undergoing percutaneous coronary intervention: 3-year clinical outcomes. J Clin Med. 2020;9(8):2464. doi: 10.3390/jcm9082464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Belle E., Périé M., Braune D., et al. Effects of coronary stenting on vessel patency and long-term clinical outcome after percutaneous coronary revascularization in diabetic patients. J Am Coll Cardiol. 2002;40(3):410–417. doi: 10.1016/s0735-1097(02)01971-x. [DOI] [PubMed] [Google Scholar]
- 5.Koskinas K.C., Siontis G.C.M., Piccolo R., et al. Impact of diabetic status on outcomes after revascularization with drug-eluting stents in relation to coronary artery disease complexity: patient-level pooled analysis of 6081 patients. Circ Cardiovasc Interv. 2016;9(2) doi: 10.1161/circinterventions.115.003255. [DOI] [PubMed] [Google Scholar]
- 6.Nicholls S.J., Tuzcu E.M., Kalidindi S., et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 2008;52(4):255–262. doi: 10.1016/j.jacc.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 7.Beckman J.A., Creager M.A., Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 8.Yazbek N., Bapat A., Kleiman N. Platelet abnormalities in diabetes mellitus. Coron Artery Dis. 2003;14(5):365–371. doi: 10.1097/01.mca.0000085138.16622.9e. [DOI] [PubMed] [Google Scholar]
- 9.Lin J., Thompson T.J., Cheng Y.J., et al. Projection of the future diabetes burden in the United States through 2060. Popul Health Metr. 2018;16(1):9. doi: 10.1186/s12963-018-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(3):e18–e114. doi: 10.1161/CIR.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 11.Byrne R.A., Rossello X., Coughlan J.J., et al. 2023 ESC Guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC) Eur Heart J. 2023;44(38):3720–3826. doi: 10.1093/eurheartj/ehad191. [DOI] [PubMed] [Google Scholar]
- 12.Centre for Open Science . 2023. Comparison of different durations of dual antiplatelet therapy after percutaneous coronary intervention in diabetes mellitus: a systematic review and network meta-analysis. [DOI] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park D.Y., Wang P., An S., et al. Shortening the duration of dual antiplatelet therapy after percutaneous coronary intervention for acute coronary syndrome: a systematic review and meta-analysis. Am Heart J. 2022;251:101–114. doi: 10.1016/j.ahj.2022.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber F., Knapp G., Ickstadt K., Kundt G., Glass Ä. Zero-cell corrections in random-effects meta-analyses. Res Synth Methods. 2020;11(6):913–919. doi: 10.1002/jrsm.1460. [DOI] [PubMed] [Google Scholar]
- 18.Rücker G., Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Luca G., Damen S.A., Camaro C., et al. Final results of the randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with a new-generation stent (REDUCE trial) EuroIntervention. 2019;15(11):e990–e998. doi: 10.4244/EIJ-D-19-00539. [DOI] [PubMed] [Google Scholar]
- 20.Franzone A., McFadden E., Leonardi S., et al. Ticagrelor alone versus dual antiplatelet therapy from 1 month after drug-eluting coronary stenting. J Am Coll Cardiol. 2019;74(18):2223–2234. doi: 10.1016/j.jacc.2019.08.1038. [DOI] [PubMed] [Google Scholar]
- 21.Gwon H.C., Hahn J.Y., Park K.W., et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125(3):505–513. doi: 10.1161/CIRCULATIONAHA.111.059022. [DOI] [PubMed] [Google Scholar]
- 22.Hahn J.Y., Song Y.B., Oh J.H., et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA. 2019;321(24):2428–2437. doi: 10.1001/jama.2019.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han J.K., Hwang D., Yang S., et al. Comparison of 3- to 6-month versus 12-month dual antiplatelet therapy after coronary intervention using the contemporary drug-eluting stents with ultrathin struts: the HOST-IDEA randomized clinical trial. Circulation. 2023;147(18):1358–1368. doi: 10.1161/CIRCULATIONAHA.123.064264. [DOI] [PubMed] [Google Scholar]
- 24.Han Y., Xu B., Xu K., et al. Six versus 12 months of dual antiplatelet therapy after implantation of biodegradable polymer sirolimus-eluting stent: randomized substudy of the I-LOVE-IT 2 trial. Circ Cardiovasc Interv. 2016;9(2) doi: 10.1161/CIRCINTERVENTIONS.115.003145. [DOI] [PubMed] [Google Scholar]
- 25.Hong S.J., Shin D.H., Kim J.S., et al. 6-month versus 12-month dual-antiplatelet therapy following long everolimus-eluting stent implantation. JACC Cardiovasc Interv. 2016;9(14):1438–1446. doi: 10.1016/j.jcin.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 26.Kim B.K., Hong S.J., Cho Y.H., et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome. JAMA. 2020;323(23):2407–2416. doi: 10.1001/jama.2020.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim B.K., Hong M.K., Shin D.H., et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation) J Am Coll Cardiol. 2012;60(15):1340–1348. doi: 10.1016/j.jacc.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 28.Mehran R., Baber U., Sharma S.K., et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381(21):2032–2042. doi: 10.1056/NEJMoa1908419. [DOI] [PubMed] [Google Scholar]
- 29.Schulz-Schüpke S., Byrne R.A., Ten Berg J.M., et al. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J. 2015;36(20):1252–1263. doi: 10.1093/eurheartj/ehu523. [DOI] [PubMed] [Google Scholar]
- 30.Valgimigli M., Frigoli E., Heg D., et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med. 2021;385(18):1643–1655. doi: 10.1056/NEJMoa2108749. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe H., Domei T., Morimoto T., et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. 2019;321(24):2414–2427. doi: 10.1001/jama.2019.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colombo A., Chieffo A., Frasheri A., et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64(20):2086–2097. doi: 10.1016/j.jacc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Feres F., Costa R.A., Abizaid A., et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310(23):2510–2522. doi: 10.1001/jama.2013.282183. [DOI] [PubMed] [Google Scholar]
- 34.Hahn J.Y., Song Y.B., Oh J.H., et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. 2018;391(10127):1274–1284. doi: 10.1016/S0140-6736(18)30493-8. [DOI] [PubMed] [Google Scholar]
- 35.Feres F., Costa R., Bhatt D., et al. Impact of short- versus long-term DAPT in patients with diabetes mellitus undergoing percutaneous intervention with Endeavor zotarolimus-eluting stents – A subanalysis of the large, prospective randomized, multicenter OPTIMIZE trial. J Am Coll Cardiol. 2014;63(12_Supplement):A1859. doi: 10.1016/S0735-1097(14)61862-3. [DOI] [Google Scholar]
- 36.Watanabe H., Morimoto T., Natsuaki M., et al. Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet therapy in patients with acute coronary syndrome: the STOPDAPT-2 ACS randomized clinical trial. JAMA Cardiol. 2022;7(4):407–417. doi: 10.1001/jamacardio.2021.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vranckx P., Valgimigli M., Jüni P., et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392(10151):940–949. doi: 10.1016/S0140-6736(18)31858-0. [DOI] [PubMed] [Google Scholar]
- 38.Hong S.J., Shin D.H., Kim J.S., et al. 6-month versus 12-month dual-antiplatelet therapy following long everolimus-eluting stent implantation: The IVUS-XPL randomized clinical trial. JACC Cardiovasc Interv. 2016;9(14):1438–1446. doi: 10.1016/j.jcin.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 39.Cutlip D.E., Windecker S., Mehran R., et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 40.Gargiulo G., Windecker S., da Costa B.R., et al. Short term versus long term dual antiplatelet therapy after implantation of drug eluting stent in patients with or without diabetes: systematic review and meta-analysis of individual participant data from randomised trials. BMJ. 2016;355:i5483. doi: 10.1136/bmj.i5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H., Ke J., Huang J., Xu K., Chen Y. Short- versus long-term dual antiplatelet therapy after second-generation drug-eluting stent implantation in patients with diabetes mellitus: a meta-analysis of randomized controlled trials. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0242845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma A., Garg A., Elmariah S., et al. Duration of dual antiplatelet therapy following drug-eluting stent implantation in diabetic and non-diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Prog Cardiovasc Dis. 2018;60(4-5):500–507. doi: 10.1016/j.pcad.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 43.An K., Guo P., Qiu S., et al. Optimal duration of dual antiplatelet therapy followed by monotherapy in diabetic patients after percutaneous coronary intervention with drug-eluting stent implantation: a Bayesian network meta-analysis. Pol Arch Intern Med. 2021;131(9):781–789. doi: 10.20452/pamw.16032. [DOI] [PubMed] [Google Scholar]
- 44.Kakouros N., Rade J.J., Kourliouros A., Resar J.R. Platelet function in patients with diabetes mellitus: from a theoretical to a practical perspective. Int J Endocrinol. 2011;2011 doi: 10.1155/2011/742719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angiolillo D.J., Bernardo E., Ramirez C., et al. Insulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatment. J Am Coll Cardiol. 2006;48(2):298–304. doi: 10.1016/j.jacc.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 46.Fejes Z., Póliska S., Czimmerer Z., et al. Hyperglycaemia suppresses microRNA expression in platelets to increase P2RY12 and SELP levels in type 2 diabetes mellitus. Thromb Haemost. 2017;117(03):529–542. doi: 10.1160/th16-04-0322. [DOI] [PubMed] [Google Scholar]
- 47.Kim H.K., Kim J.E., Park S.H., Kim Y.I., Nam-Goong I.S., Kim E.S. High coagulation factor levels and low protein C levels contribute to enhanced thrombin generation in patients with diabetes who do not have macrovascular complications. J Diabetes Complications. 2014;28(3):365–369. doi: 10.1016/j.jdiacomp.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Montalescot G., Brieger D., Dalby A.J., Park S.J., Mehran R. Duration of dual antiplatelet therapy after coronary stenting: a review of the evidence. J Am Coll Cardiol. 2015;66(7):832–847. doi: 10.1016/j.jacc.2015.05.053. [DOI] [PubMed] [Google Scholar]
- 49.Yeh R.W., Secemsky E.A., Kereiakes D.J., et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315(16):1735–1749. doi: 10.1001/jama.2016.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ang L., Palakodeti V., Khalid A., et al. Elevated plasma fibrinogen and diabetes mellitus are associated with lower inhibition of platelet reactivity with clopidogrel. J Am Coll Cardiol. 2008;52(13):1052–1059. doi: 10.1016/j.jacc.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 51.Angiolillo D.J., Jakubowski J.A., Ferreiro J.L., et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol. 2014;64(10):1005–1014. doi: 10.1016/j.jacc.2014.06.1170. [DOI] [PubMed] [Google Scholar]
- 52.Wrishko R.E., Ernest C.S., 2nd, Small D.S., et al. Population pharmacokinetic analyses to evaluate the influence of intrinsic and extrinsic factors on exposure of prasugrel active metabolite in TRITON-TIMI 38. J Clin Pharmacol. 2009;49(8):984–998. doi: 10.1177/0091270009337942. [DOI] [PubMed] [Google Scholar]
- 53.VAN Giezen J.J., Nilsson L., Berntsson P., et al. Ticagrelor binds to human P2Y(12) independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost. 2009;7(9):1556–1565. doi: 10.1111/j.1538-7836.2009.03527.x. [DOI] [PubMed] [Google Scholar]
- 54.Wiviott S.D., Braunwald E., Angiolillo D.J., et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118(16):1626–1636. doi: 10.1161/circulationaha.108.791061. [DOI] [PubMed] [Google Scholar]
- 55.James S., Angiolillo D.J., Cornel J.H., et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010;31(24):3006–3016. doi: 10.1093/eurheartj/ehq325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.