Abstract
The Escherichia coli gene for homoserine kinase (thrB) has been cloned into a simian-virus-40-based eukaryotic expression vector which also includes a neomycin-resistance gene. Mouse 3T3 cells transfected with this plasmid were selected for resistance and screened for homoserine kinase activity. It has thus been possible to isolate clones which are capable of accumulating homoserine O-phosphate when supplied with homoserine. In broken-cell preparations the kinetic constants for the production of homoserine O-phosphate were similar to those of the wild-type E. coli enzyme. These experiments demonstrate that E. coli homoserine kinase can be expressed in an animal cell and that it can successfully phosphorylate L-homoserine in the intact cell utilizing endogenous ATP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M. E. Similarity between human retinoic acid receptor and Escherichia coli homoserine kinase. Biochem J. 1988 Oct 15;255(2):748–749. [PMC free article] [PubMed] [Google Scholar]
- Burr B., Walker J., Truffa-Bachi P., Cohen G. N. Homoserine kinase from Escherichia coli K12. Eur J Biochem. 1976 Mar 1;62(3):519–526. doi: 10.1111/j.1432-1033.1976.tb10186.x. [DOI] [PubMed] [Google Scholar]
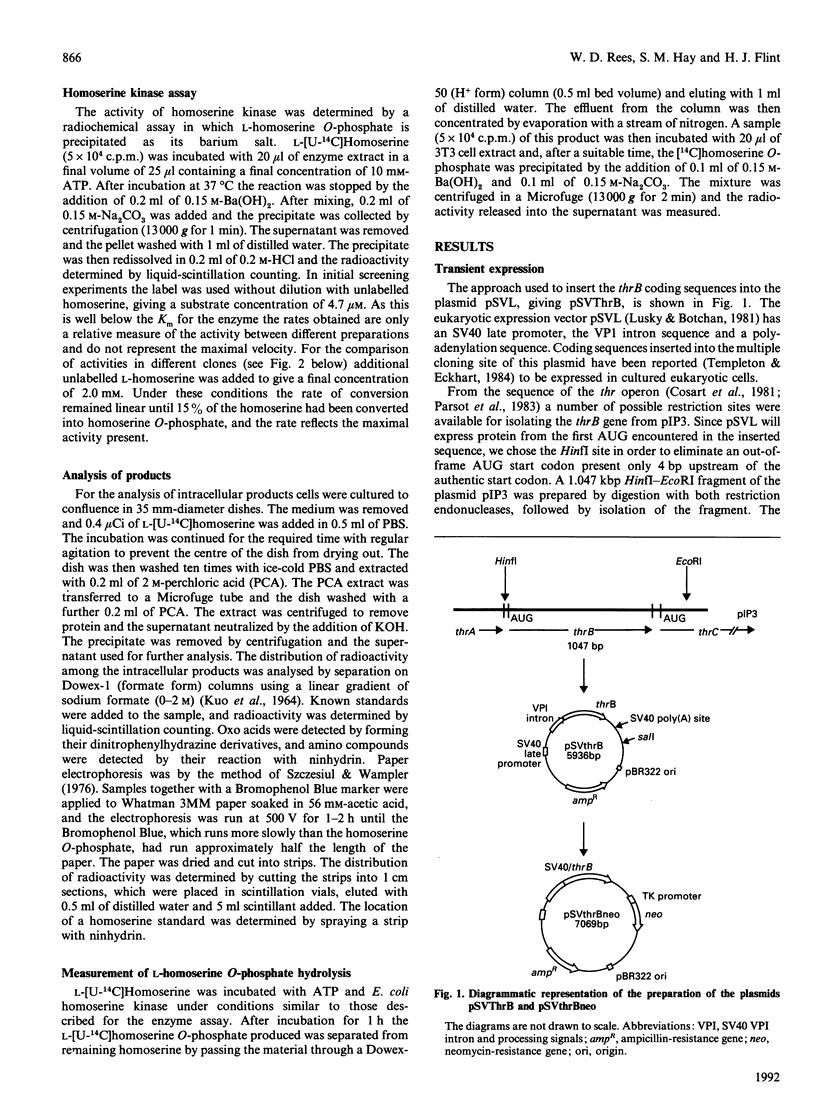
- Cossart P., Katinka M., Yaniv M. Nucleotide sequence of the thrB gene of E. coli, and its two adjacent regions; the thrAB and thrBC junctions. Nucleic Acids Res. 1981 Jan 24;9(2):339–347. doi: 10.1093/nar/9.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deme D., Chatagner F. Etude du centre actif de l'homosérine déhydratase du foie de rat. Biochim Biophys Acta. 1972 Feb 28;258(2):643–654. doi: 10.1016/0005-2744(72)90256-2. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Berg P. Construction, propagation and expression of simian virus 40 recombinant genomes containing the Escherichia coli gene for thymidine kinase and a Saccharomyces cerevisae gene for tyrosine transfer RNA. J Mol Biol. 1979 Sep 25;133(3):359–383. doi: 10.1016/0022-2836(79)90398-x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh J. E., Campbell G. P., Whitelaw P. F. c-myc mRNA in cytoskeletal-bound polysomes in fibroblasts. Biochem J. 1991 Mar 1;274(Pt 2):607–609. doi: 10.1042/bj2740607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUO M. H., SAUNDERS P. P., BROQUIST H. P. LYSINE BIOSYNTHESIS IN YEAST: A NEW METABOLITE OF ALPHA-AMINOADIPIC ACID. J Biol Chem. 1964 Feb;239:508–515. [PubMed] [Google Scholar]
- Langner K. D., Weyer U., Doerfler W. Trans effect of the E1 region of adenoviruses on the expression of a prokaryotic gene in mammalian cells: resistance to 5' -CCGG- 3' methylation. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1598–1602. doi: 10.1073/pnas.83.6.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Miyajima R., Shio I. Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum. V. Properties of homoserine kinase. J Biochem. 1972 Feb;71(2):219–226. doi: 10.1093/oxfordjournals.jbchem.a129758. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsot C., Cossart P., Saint-Girons I., Cohen G. N. Nucleotide sequence of thrC and of the transcription termination region of the threonine operon in Escherichia coli K12. Nucleic Acids Res. 1983 Nov 11;11(21):7331–7345. doi: 10.1093/nar/11.21.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. D., Flint H. J., Fuller M. F. A molecular biological approach to reducing dietary amino acid needs. Biotechnology (N Y) 1990 Jul;8(7):629–633. doi: 10.1038/nbt0790-629. [DOI] [PubMed] [Google Scholar]
- Schümperli D., Howard B. H., Rosenberg M. Efficient expression of Escherichia coli galactokinase gene in mammalian cells. Proc Natl Acad Sci U S A. 1982 Jan;79(2):257–261. doi: 10.1073/pnas.79.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Szczesiul M., Wampler D. E. Regulation of a metabolic system in vitro: synthesis of threonine from aspartic acid. Biochemistry. 1976 May 18;15(10):2236–2244. doi: 10.1021/bi00655a033. [DOI] [PubMed] [Google Scholar]
- Templeton D., Eckhart W. N-terminal amino acid sequences of the polyoma middle-size T antigen are important for protein kinase activity and cell transformation. Mol Cell Biol. 1984 May;4(5):817–821. doi: 10.1128/mcb.4.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théze J., Kleidman L., St Girons I. Homoserine kinase from Escherichia coli K-12: properties, inhibition by L-threonine, and regulation of biosynthesis. J Bacteriol. 1974 May;118(2):577–581. doi: 10.1128/jb.118.2.577-581.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware C. E., Bauchop T., Gregg K. The isolation and comparison of cellulase genes from two strains of Ruminococcus albus. J Gen Microbiol. 1989 Apr;135(4):921–930. doi: 10.1099/00221287-135-4-921. [DOI] [PubMed] [Google Scholar]