Abstract
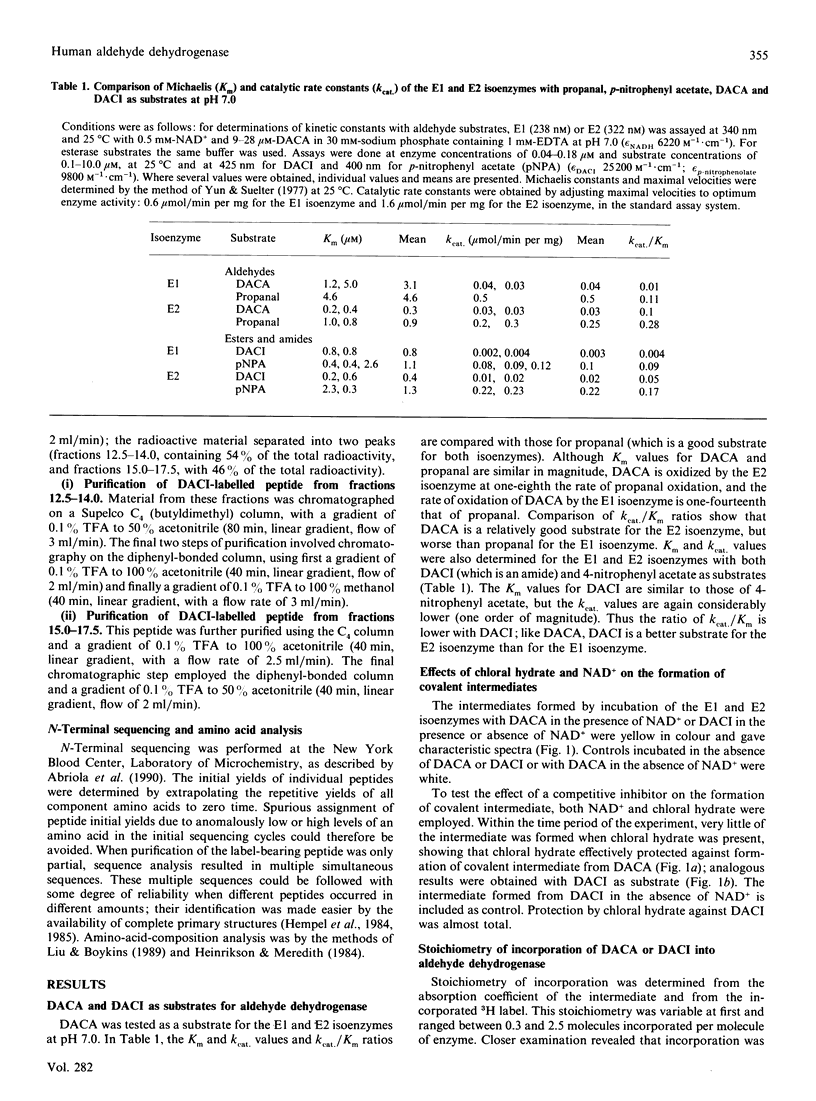
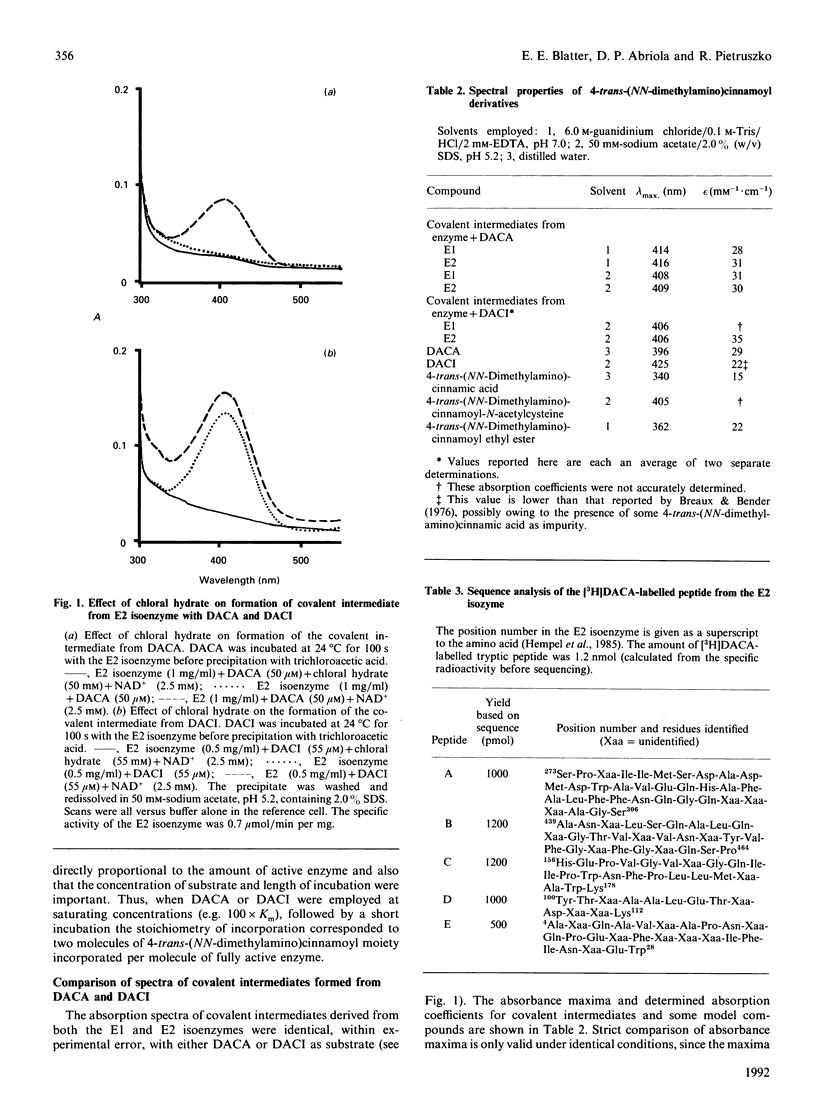
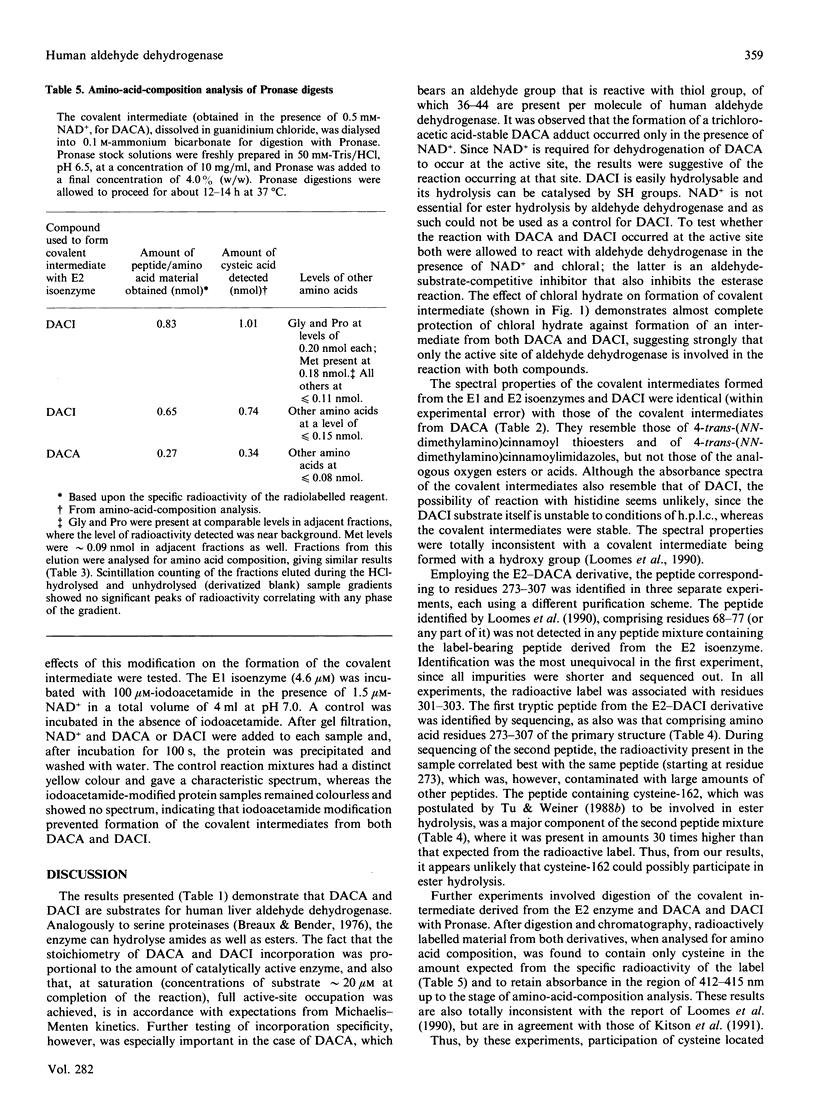
4-trans-(NN-Dimethylamino)cinnamaldehyde (an aldehyde, DACA) and 4-trans-(NN-dimethylamino)cinnamoylimidazole (an amide, DACI) have been shown to be substrates for human aldehyde dehydrogenase (EC 1.2.1.3) which form chromophoric covalent intermediates. The spectra of covalent intermediates from both the cytoplasmic (E1) and mitochondrial (E2) isoenzymes derived from DACA and DACI were compared. The spectra were similar when either substrate was used, and also when the two isoenzymes were compared, and resembled that obtained for 4-trnas-(NN-dimethylamino)cinnamoyl-N-acetylcysteine, but differed from the spectrum of 4-trans-(NN-dimethylamino)cinnamoyl ethyl ester. After extensive digestion of the covalent intermediates from both 3H-labelled DACA and DACI with Pronase and purification, the labelled amino acid was identified as cysteine. Covalent intermediates from both DACA and DACI were also digested with trypsin, and labelled peptides were purified by ion-exchange and reverse-phase chromatography. Amino acid sequence analysis showed that the peptide comprising residues 273-307 was labelled by both DACA and DACI. The radioactive label at cysteine residues 301-303 of the primary structure could be unequivocally identified by employing the DACA derivative. Assignment of label to cysteine-302 was achieved by employing iodoacetamide-labelled E1 isoenzyme (iodoacetamide specifically labels cysteine-302), in which case there was no formation of the covalent intermediate from either DACA or DACI. In addition, cysteine-302 is the only cysteine residue conserved in all aldehyde dehydrogenases sequenced. Thus cysteine-302 is the amino acid residue that forms a covalent intermediate with both aldehyde and ester substrates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abriola D. P., Fields R., Stein S., MacKerell A. D., Jr, Pietruszko R. Active site of human liver aldehyde dehydrogenase. Biochemistry. 1987 Sep 8;26(18):5679–5684. doi: 10.1021/bi00392a015. [DOI] [PubMed] [Google Scholar]
- Abriola D. P., MacKerell A. D., Jr, Pietruszko R. Correlation of loss of activity of human aldehyde dehydrogenase with reaction of bromoacetophenone with glutamic acid-268 and cysteine-302 residues. Partial-sites reactivity of aldehyde dehydrogenase. Biochem J. 1990 Feb 15;266(1):179–187. doi: 10.1042/bj2660179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter E. E., Tasayco M. L., Prestwich G., Pietruszko R. Chemical modification of aldehyde dehydrogenase by a vinyl ketone analogue of an insect pheromone. Biochem J. 1990 Dec 1;272(2):351–358. doi: 10.1042/bj2720351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breaux E. J., Bender M. L. Direct spectrophotometric observation of an acyl-enzyme intermediate in elastase catalysis. Biochem Biophys Res Commun. 1976 May 3;70(1):235–240. doi: 10.1016/0006-291x(76)91133-5. [DOI] [PubMed] [Google Scholar]
- Buckley P. D., Dunn M. F. Observation of acyl-enzyme intermediates in the sheep liver aldehyde dehydrogenase catalytic mechanism via rapid-scanning UV-visible spectroscopy. Prog Clin Biol Res. 1982;114:23–35. [PubMed] [Google Scholar]
- Deady L. W., Buckley P. D., Bennett A. F., Blackwell L. F. Kinetics of inhibition and hysteresis of sheep liver cytoplasmic aldehyde dehydrogenase with glyoxylic acid: further evidence relating to the two-site model for aldehyde oxidation. Arch Biochem Biophys. 1985 Dec;243(2):586–597. doi: 10.1016/0003-9861(85)90536-3. [DOI] [PubMed] [Google Scholar]
- Duncan R. J. Aldehyde dehydrogenase. An enzyme with two distinct catalytic activities at a single type of active site. Biochem J. 1985 Aug 15;230(1):261–267. doi: 10.1042/bj2300261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R. J. Reversal of part of the aldehyde dehydrogenase reaction pathway during the hydrolysis of an ester. Biochem J. 1979 Nov 1;183(2):459–462. doi: 10.1042/bj1830459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M. F., Buckley P. D. Kinetic and spectroscopic characterization of the sheep liver aldehyde dehydrogenase acyl-enzyme. Prog Clin Biol Res. 1985;174:15–27. [PubMed] [Google Scholar]
- Greenfield N. J., Pietruszko R. Two aldehyde dehydrogenases from human liver. Isolation via affinity chromatography and characterization of the isozymes. Biochim Biophys Acta. 1977 Jul 8;483(1):35–45. doi: 10.1016/0005-2744(77)90005-5. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Hempel J. D., Pietruszko R. Selective chemical modification of human liver aldehyde dehydrogenases E1 and E2 by iodoacetamide. J Biol Chem. 1981 Nov 10;256(21):10889–10896. [PubMed] [Google Scholar]
- Hempel J. D., Reed D. M., Pietruszko R. Human aldehyde dehydrogenase: improved purification procedure and comparison of homogeneous isoenzymes E1 and E2. Alcohol Clin Exp Res. 1982 Summer;6(3):417–425. doi: 10.1111/j.1530-0277.1982.tb05001.x. [DOI] [PubMed] [Google Scholar]
- Hempel J., Kaiser R., Jörnvall H. Mitochondrial aldehyde dehydrogenase from human liver. Primary structure, differences in relation to the cytosolic enzyme, and functional correlations. Eur J Biochem. 1985 Nov 15;153(1):13–28. doi: 10.1111/j.1432-1033.1985.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Hempel J., Pietruszko R., Fietzek P., Jörnvall H. Identification of a segment containing a reactive cysteine residue in human liver cytoplasmic aldehyde dehydrogenase (isoenzyme E1). Biochemistry. 1982 Dec 21;21(26):6834–6838. doi: 10.1021/bi00269a032. [DOI] [PubMed] [Google Scholar]
- Hempel J., von Bahr-Lindström H., Jörnvall H. Aldehyde dehydrogenase from human liver. Primary structure of the cytoplasmic isoenzyme. Eur J Biochem. 1984 May 15;141(1):21–35. doi: 10.1111/j.1432-1033.1984.tb08150.x. [DOI] [PubMed] [Google Scholar]
- Kitson T. M., Hill J. P., Midwinter G. G. Identification of a catalytically essential nucleophilic residue in sheep liver cytoplasmic aldehyde dehydrogenase. Biochem J. 1991 Apr 1;275(Pt 1):207–210. doi: 10.1042/bj2750207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson T. M. Studies on the interaction between disulfiram and sheep liver cytoplasmic aldehyde dehydrogenase. Biochem J. 1978 Oct 1;175(1):83–90. doi: 10.1042/bj1750083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. Y., Boykins R. A. Hydrolysis of proteins and peptides in a hermetically sealed microcapillary tube: high recovery of labile amino acids. Anal Biochem. 1989 Nov 1;182(2):383–387. doi: 10.1016/0003-2697(89)90612-x. [DOI] [PubMed] [Google Scholar]
- Loomes K. M., Kitson T. M. Aldehyde dehydrogenase catalyses acetaldehyde formation from 4-nitrophenyl acetate and NADH. Biochem J. 1986 Sep 1;238(2):617–619. doi: 10.1042/bj2380617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes K. M., Midwinter G. G., Blackwell L. F., Buckley P. D. Evidence for reactivity of serine-74 with trans-4-(N,N-dimethylamino)cinnamaldehyde during oxidation by the cytoplasmic aldehyde dehydrogenase from sheep liver. Biochemistry. 1990 Feb 27;29(8):2070–2075. doi: 10.1021/bi00460a015. [DOI] [PubMed] [Google Scholar]
- MacGibbon A. K., Haylock S. J., Buckley P. D., Blackwell L. F. Kinetic studies on the esterase activity of cytoplasmic sheep liver aldehyde dehydrogenase. Biochem J. 1978 Jun 1;171(3):533–538. doi: 10.1042/bj1710533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKerell A. D., Jr, MacWright R. S., Pietruszko R. Bromoacetophenone as an affinity reagent for human liver aldehyde dehydrogenase. Biochemistry. 1986 Sep 9;25(18):5182–5189. doi: 10.1021/bi00366a030. [DOI] [PubMed] [Google Scholar]
- Tu G. C., Weiner H. Evidence for two distinct active sites on aldehyde dehydrogenase. J Biol Chem. 1988 Jan 25;263(3):1218–1222. [PubMed] [Google Scholar]
- Tu G. C., Weiner H. Identification of the cysteine residue in the active site of horse liver mitochondrial aldehyde dehydrogenase. J Biol Chem. 1988 Jan 25;263(3):1212–1217. [PubMed] [Google Scholar]
- Weber J. A., Turpin P., Bernhard S. A., Peticolas W. L. Chromophoric cinnamic acid substrates as resonance Raman probes of the active site environment in native and unfolded alpha-chymotrypsin. Biochemistry. 1986 Apr 22;25(8):1912–1917. doi: 10.1021/bi00356a012. [DOI] [PubMed] [Google Scholar]
- Yun S. L., Suelter C. H. A simple method for calculating Km and V from a single enzyme reaction progress curve. Biochim Biophys Acta. 1977 Jan 11;480(1):1–13. doi: 10.1016/0005-2744(77)90315-1. [DOI] [PubMed] [Google Scholar]
- von Bahr-Lindström H., Jeck R., Woenckhaus C., Sohn S., Hempel J., Jörnvall H. Characterization of the coenzyme binding site of liver aldehyde dehydrogenase: differential reactivity of coenzyme analogues. Biochemistry. 1985 Oct 8;24(21):5847–5851. doi: 10.1021/bi00342a023. [DOI] [PubMed] [Google Scholar]


