Abstract
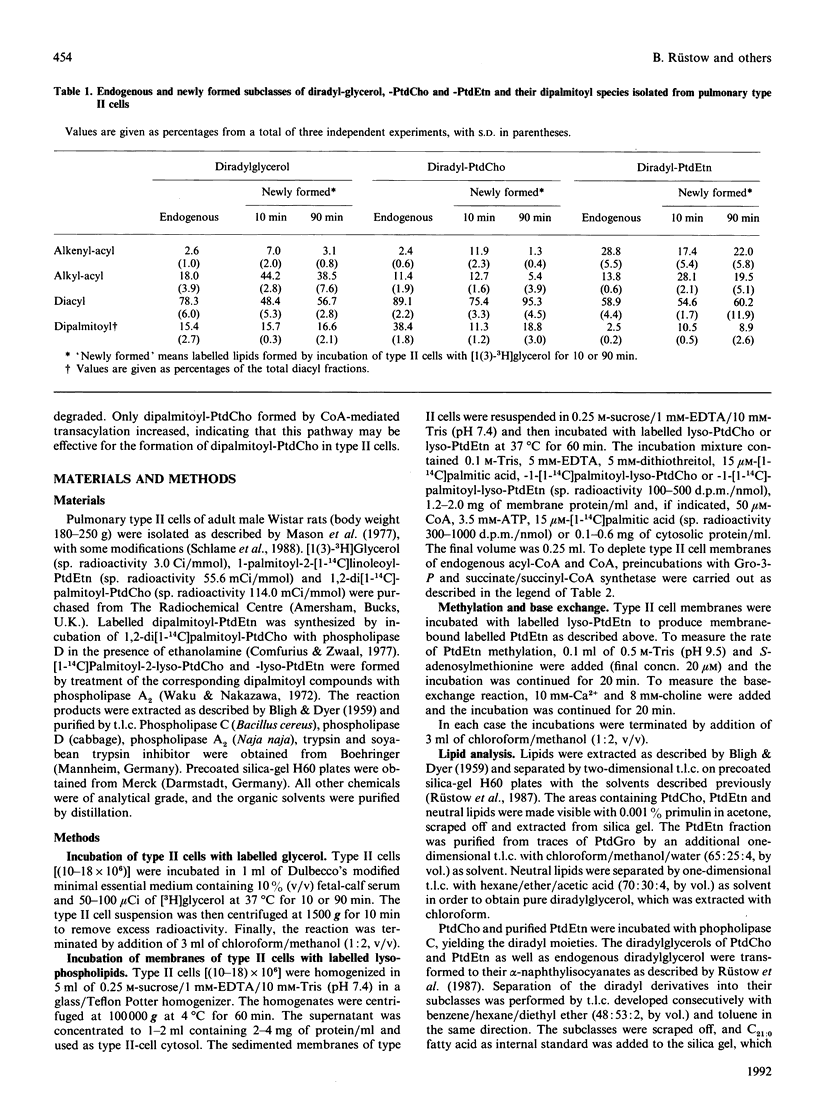
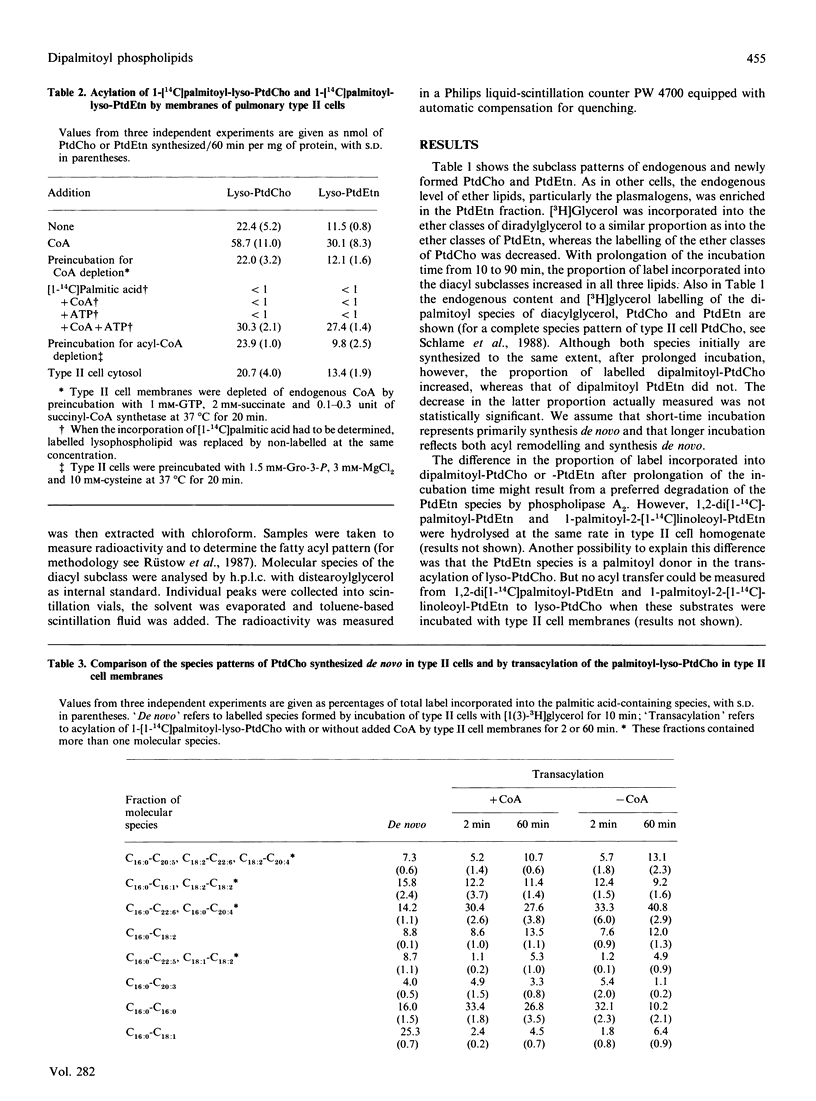
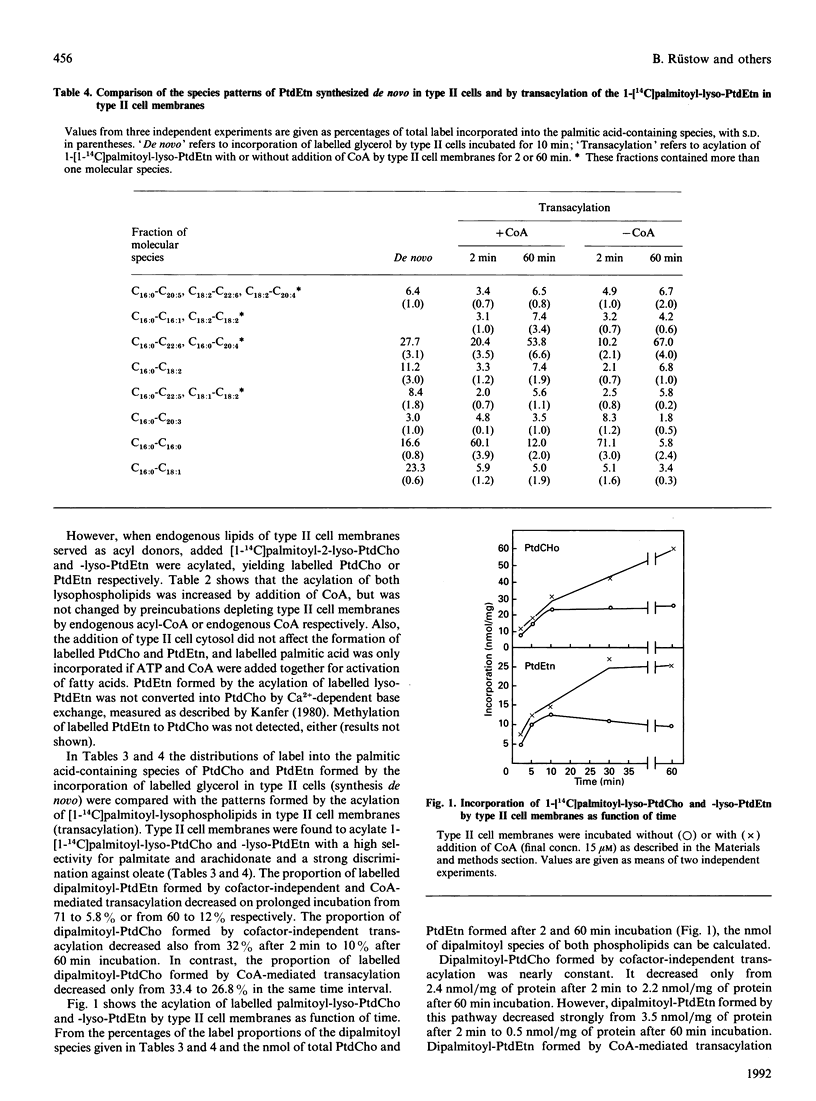
Endogenous content of and incorporation of labelled glycerol into alkenylacyl-, alkylacyl- and diacyl-glycerol, -glycerol-3-phosphocholine and -glycero-3-phosphoethanolamine of pulmonary type II cells were measured. On prolonged incubation of type II cells with labelled glycerol, the proportion of label incorporated into the diacyl subclass of these glycerolipids increased and the proportion of label incorporated into the ether lipids declined. Endogenous phosphatidylcholine (PtdCho) of type II cells contained 38.4% of the dipalmitoyl species, but endogenous phosphatidylethanolamine (PtdEtn) only 2.5%. In contrast, similar proportions of labelled glycerol were incorporated into dipalmitoyl-PtdCho and -PtdEtn after short-time incubation but, with prolonged incubation time the proportion of labelled dipalmitoyl-PtdCho increased from 11.3 to 18.8%, whereas that of dipalmitoyl-PtdEtn did not change significantly. Type II cell membranes were found to exhibit cofactor-independent and CoA-mediated transacylations of [1-14C]palmitoyl-lyso-PtdCho and -lyso-PtdEtn. The distribution of label among the palmitic acid-containing species of PtdCho and PtdEtn formed by both transacylation activities was determined. Cofactor-independent and CoA-mediated transacylation showed a strong selectivity for palmitate and arachidonate and a strong discrimination against oleate. The amount (nmol) of dipalmitoyl-PtdEtn formed by both transacylation activities after short-time incubation (2 min) decreased with prolonged incubation time (60 min). In contrast, the nmol of dipalmitoyl-PtdCho formed by cofactor-independent transacylation remains nearly the same after short-time and longer incubation. The nmol of dipalmitoyl-PtdCho formed by CoA-mediated transacylation increased strongly in the same time interval. Beside synthesis de novo via the CDP-choline pathway and reacylation of lyso-PtdCho with palmitoyl-CoA, the CoA-mediated transacylation of lyso-PtdCho may be an effective pathway for the formation of dipalmitoyl-PtdCho in pulmonary type II cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Binaglia L., Roberti R., Vecchini A., Porcellati G. Evidence for a compartmentation of brain microsomal diacylglycerol. J Lipid Res. 1982 Sep;23(7):955–961. [PubMed] [Google Scholar]
- Comfurius P., Zwaal R. F. The enzymatic synthesis of phosphatidylserine and purification by CM-cellulose column chromatography. Biochim Biophys Acta. 1977 Jul 20;488(1):36–42. doi: 10.1016/0005-2760(77)90120-5. [DOI] [PubMed] [Google Scholar]
- Crecelius C. A., Longmore W. J. A study of the molecular species of diacylglycerol, phosphatidylcholine and phosphatidylethanolamine and of cholinephosphotransferase and ethanolaminephosphotransferase activities in the type II pneumocyte. Biochim Biophys Acta. 1984 Sep 12;795(2):247–256. doi: 10.1016/0005-2760(84)90072-9. [DOI] [PubMed] [Google Scholar]
- Crecelius C. A., Longmore W. J. Acyltransferase activities in adult rat type II pneumocyte-derived subcellular fractions. Biochim Biophys Acta. 1984 Sep 12;795(2):238–246. doi: 10.1016/0005-2760(84)90071-7. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Huber G. A., Furia L., Bassett D., Rabinowitz J. L. Evidence for lipid synthesis by the dihydroxyacetone phosphate pathway in rabbit lung subcellular fractions. J Lab Clin Med. 1976 Jun;87(6):1033–1040. [PubMed] [Google Scholar]
- Friedberg S. J., Heifetz A. Hydrogen exchange in the synthesis of glyceryl ether and in the formation of dihydroxyacetone in Tetrahymena pyriformis. Biochemistry. 1973 Mar 13;12(6):1100–1106. doi: 10.1021/bi00730a013. [DOI] [PubMed] [Google Scholar]
- Goerke J. Lung surfactant. Biochim Biophys Acta. 1974 Dec 16;344(3-4):241–261. doi: 10.1016/0304-4157(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Hallman M., Enhorning G., Possmayer F. Composition and surface activity of normal and phosphatidylglycerol-deficient lung surfactant. Pediatr Res. 1985 Mar;19(3):286–292. doi: 10.1203/00006450-198503000-00006. [DOI] [PubMed] [Google Scholar]
- Hirai K., Yamauchi M., Witschi H., Côté M. G. Disintegration of lung peroxisomes during differentiation of type II cells to type I cells in butylated hydroxytoluene-administered mice. Exp Mol Pathol. 1983 Oct;39(2):129–138. doi: 10.1016/0014-4800(83)90046-1. [DOI] [PubMed] [Google Scholar]
- Kanfer J. N. The base exchange enzymes and phospholipase D of mammalian tissue. Can J Biochem. 1980 Dec;58(12):1370–1380. doi: 10.1139/o80-186. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Deykin D. Arachidonoyl transacylase in human platelets. Coenzyme A-independent transfer of arachidonate from phosphatidylcholine to lysoplasmenylethanolamine. J Biol Chem. 1983 Nov 25;258(22):13806–13811. [PubMed] [Google Scholar]
- Mason R. J., Dobbs L. G. Synthesis of phosphatidylcholine and phosphatidylglycerol by alveolar type II cells in primary culture. J Biol Chem. 1980 Jun 10;255(11):5101–5107. [PubMed] [Google Scholar]
- Mason R. J. Importance of the acyl dihydroxyacetone phosphate pathway in the synthesis of phosphatidylglycerol and phosphatidylcholine in alveolar type II cells. J Biol Chem. 1978 May 25;253(10):3367–3370. [PubMed] [Google Scholar]
- Mason R. J., Williams M. C., Greenleaf R. D., Clements J. A. Isolation and properties of type II alveolar cells from rat lung. Am Rev Respir Dis. 1977 Jun;115(6):1015–1026. doi: 10.1164/arrd.1977.115.6.1015. [DOI] [PubMed] [Google Scholar]
- Nijssen J. G., van den Bosch H. Coenzyme A-mediated transacylation of sn-2 fatty acids from phosphatidylcholine in rat lung microsomes. Biochim Biophys Acta. 1986 Feb 28;875(3):458–464. doi: 10.1016/0005-2760(86)90065-2. [DOI] [PubMed] [Google Scholar]
- Nijssen J. G., van den Bosch H. Cytosol-stimulated remodeling of phosphatidylcholine in rat lung microsomes. Biochim Biophys Acta. 1986 Feb 28;875(3):450–457. doi: 10.1016/0005-2760(86)90064-0. [DOI] [PubMed] [Google Scholar]
- Post M., Schuurmans E. A., Batenburg J. J., Van Golde L. M. Mechanisms involved in the synthesis of disaturated phosphatidylcholine by alveolar type II cells isolated from adult rat lung. Biochim Biophys Acta. 1983 Jan 7;750(1):68–77. doi: 10.1016/0005-2760(83)90205-9. [DOI] [PubMed] [Google Scholar]
- Post M., van Golde L. M. Metabolic and developmental aspects of the pulmonary surfactant system. Biochim Biophys Acta. 1988 Jun 9;947(2):249–286. doi: 10.1016/0304-4157(88)90011-1. [DOI] [PubMed] [Google Scholar]
- Rüstow B., Kunze D. Diacylglycerol synthesized in vitro from sn-glycerol 3-phosphate and the endogenous diacylglycerol are different substrate pools for the biosynthesis of phosphatidylcholine in rat lung microsomes. Biochim Biophys Acta. 1985 Jul 9;835(2):273–278. doi: 10.1016/0005-2760(85)90282-6. [DOI] [PubMed] [Google Scholar]
- Rüstow B., Kunze D. Further evidence for the existence of different diacylglycerol pools of the phosphatidylcholine synthesis in microsomes. Biochim Biophys Acta. 1987 Oct 17;921(3):552–558. doi: 10.1016/0005-2760(87)90083-x. [DOI] [PubMed] [Google Scholar]
- Rüstow B., Kunze D. The availability of endogenous and exogenous disaturated diacylglycerol for the diacylglycerol-consuming reactions in lung microsomes. Biochim Biophys Acta. 1984 Dec 6;796(3):359–363. doi: 10.1016/0005-2760(84)90138-3. [DOI] [PubMed] [Google Scholar]
- Rüstow B., Nakagawa Y., Rabe H., Waku K., Kunze D. Species pattern of phosphatidylinositol from lung surfactant and a comparison of the species pattern of phosphatidylinositol and phosphatidylglycerol synthesized de novo in lung microsomal fractions. Biochem J. 1988 Aug 15;254(1):67–71. doi: 10.1042/bj2540067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M., Casals C., Rüstow B., Rabe H., Kunze D. Molecular species of phosphatidylcholine and phosphatidylglycerol in rat lung surfactant and different pools of pneumocytes type II. Biochem J. 1988 Jul 1;253(1):209–215. doi: 10.1042/bj2530209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M., Rüstow B., Kunze D., Rabe H., Reichmann G. Phosphatidylglycerol of rat lung. Intracellular sites of formation de novo and acyl species pattern in mitochondria, microsomes and surfactant. Biochem J. 1986 Nov 15;240(1):247–252. doi: 10.1042/bj2400247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman D. M., Bell R. M. Microsomal sn-glycerol 3-phosphate and dihydroxyacetone phosphate acyltransferase activities from liver and other tissues. Evidence for a single enzyme catalizing both reactions. Arch Biochem Biophys. 1977 Aug;182(2):732–742. doi: 10.1016/0003-9861(77)90555-0. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E. Development of peroxisomes in granular pneumocytes during pre- and postnatal growth. Lab Invest. 1972 Dec;27(6):581–589. [PubMed] [Google Scholar]
- Stymne S., Stobart A. K. Involvement of acyl exchange between acyl-CoA and phosphatidylcholine in the remodelling of phosphatidylcholine in microsomal preparations of rat lung. Biochim Biophys Acta. 1985 Dec 4;837(3):239–250. doi: 10.1016/0005-2760(85)90047-5. [DOI] [PubMed] [Google Scholar]
- Van Golde L. M., Batenburg J. J., Robertson B. The pulmonary surfactant system: biochemical aspects and functional significance. Physiol Rev. 1988 Apr;68(2):374–455. doi: 10.1152/physrev.1988.68.2.374. [DOI] [PubMed] [Google Scholar]
- Waku K., Nakazawa Y. Acyltransferae activity to 1-acyl-, 1-O-alkenyl-, and 1-O-alkyl-glycero-3-phosphorylcholine in Ehrlich ascites tumor cells. J Biochem. 1972 Aug;72(2):495–497. doi: 10.1093/oxfordjournals.jbchem.a129928. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Clements J. A. Metabolism and turnover of lung surfactant. Am Rev Respir Dis. 1987 Aug;136(2):426–444. doi: 10.1164/ajrccm/136.2.426. [DOI] [PubMed] [Google Scholar]
- Wykle R. L., Malone B., Snyder F. Biosynthesis of dipalmitoyl-sn-glycero-3-phosphocholine by adenoma alveolar type II cells. Arch Biochem Biophys. 1977 May;181(1):249–256. doi: 10.1016/0003-9861(77)90503-3. [DOI] [PubMed] [Google Scholar]


