Abstract
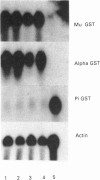
Selenium deficiency in rats for a period of up to 6 weeks inhibited both the production of 3,3',5-tri-iodothyronine (T3) from thyroxine (T4) (5'-deiodination) and also the catabolism of T3 to 3,3'-di-iodothyronine (5-deiodination) in liver homogenates. The hepatic stores of T3 were decreased by only 8% in selenium deficiency, despite the T3 production rate from T4 being only 7% of the rate found in selenium-supplemented rats. Hepatic glutathione S-transferase (GST) activity was increased in both hypothyroidism and selenium deficiency, but apparently by different mechanisms, since mRNA expression for this family of enzymes was lowered by hypothyroidism and increased in selenium deficiency. It is concluded that, since both T3 production and catabolism are inhibited by selenium deficiency, there is little change in hepatic T3 stores, and therefore the changes in the activity of certain hepatic enzymes, such as GST, that are found in selenium deficiency are not the result of tissue hypothyroidism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur J. R., Boyne R. Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci. 1985 Apr 22;36(16):1569–1575. doi: 10.1016/0024-3205(85)90381-9. [DOI] [PubMed] [Google Scholar]
- Arthur J. R., Morrice P. C., Nicol F., Beddows S. E., Boyd R., Hayes J. D., Beckett G. J. The effects of selenium and copper deficiencies on glutathione S-transferase and glutathione peroxidase in rat liver. Biochem J. 1987 Dec 1;248(2):539–544. doi: 10.1042/bj2480539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J. R., Nicol F., Beckett G. J. Hepatic iodothyronine 5'-deiodinase. The role of selenium. Biochem J. 1990 Dec 1;272(2):537–540. doi: 10.1042/bj2720537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J. R., Nicol F., Hutchinson A. R., Beckett G. J. The effects of selenium depletion and repletion on the metabolism of thyroid hormones in the rat. J Inorg Biochem. 1990 Jun;39(2):101–108. doi: 10.1016/0162-0134(90)80018-s. [DOI] [PubMed] [Google Scholar]
- Beckett G. J., Beddows S. E., Morrice P. C., Nicol F., Arthur J. R. Inhibition of hepatic deiodination of thyroxine is caused by selenium deficiency in rats. Biochem J. 1987 Dec 1;248(2):443–447. doi: 10.1042/bj2480443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett G. J., MacDougall D. A., Nicol F., Arthur R. Inhibition of type I and type II iodothyronine deiodinase activity in rat liver, kidney and brain produced by selenium deficiency. Biochem J. 1989 May 1;259(3):887–892. doi: 10.1042/bj2590887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett G. J., Nicol F., Proudfoot D., Dyson K., Loucaides G., Arthur J. R. The changes in hepatic enzyme expression caused by selenium deficiency and hypothyroidism in rats are produced by independent mechanisms. Biochem J. 1990 Mar 15;266(3):743–747. doi: 10.1042/bj2660743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behne D., Kyriakopoulos A., Meinhold H., Köhrle J. Identification of type I iodothyronine 5'-deiodinase as a selenoenzyme. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1143–1149. doi: 10.1016/s0006-291x(05)80905-2. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Larsen P. R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991 Jan 31;349(6308):438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Board P. G., Webb G. C. Isolation of a cDNA clone and localization of human glutathione S-transferase 2 genes to chromosome band 6p12. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2377–2381. doi: 10.1073/pnas.84.8.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Kano T., Sakai M., Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987 Nov 1;47(21):5626–5630. [PubMed] [Google Scholar]
- Leader D. P., Gall I., Lehrach H. The structure of a cloned mouse gamma-actin processed pseudogene. Gene. 1985;36(3):369–374. doi: 10.1016/0378-1119(85)90193-3. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Meehan R. R., Barlow D. P., Hill R. E., Hogan B. L., Hastie N. D. Pattern of serum protein gene expression in mouse visceral yolk sac and foetal liver. EMBO J. 1984 Aug;3(8):1881–1885. doi: 10.1002/j.1460-2075.1984.tb02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe W. A., Challand G. S., Ratcliffe J. G. A critical evaluation of separation methods in radiommunoassay for total triiodothyronine and thyroxine in unextracted human serum. Ann Clin Biochem. 1974 Nov;11(6):224–229. doi: 10.1177/000456327401100166. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saedi M. S., Smith C. G., Frampton J., Chambers I., Harrison P. R., Sunde R. A. Effect of selenium status on mRNA levels for glutathione peroxidase in rat liver. Biochem Biophys Res Commun. 1988 Jun 16;153(2):855–861. doi: 10.1016/s0006-291x(88)81174-4. [DOI] [PubMed] [Google Scholar]
- Safran M., Farwell A. P., Leonard J. L. Evidence that type II 5'-deiodinase is not a selenoprotein. J Biol Chem. 1991 Jul 25;266(21):13477–13480. [PubMed] [Google Scholar]
- Song M. K., Dozin B., Grieco D., Rall J. E., Nikodem V. M. Transcriptional activation and stabilization of malic enzyme mRNA precursor by thyroid hormone. J Biol Chem. 1988 Dec 5;263(34):17970–17974. [PubMed] [Google Scholar]
- Williams M. T., Carrington H., Herrera A. Stimulation of mouse liver glutathione S-transferase activity in propylthiouracil-treated mice in vivo by tri-iodothyronine. Biochem J. 1986 Jan 15;233(2):595–598. doi: 10.1042/bj2330595. [DOI] [PMC free article] [PubMed] [Google Scholar]