Abstract
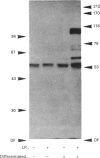
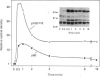
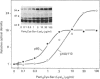
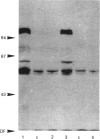
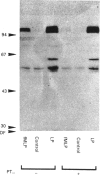
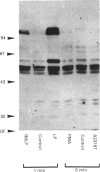
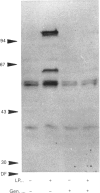
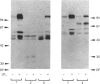
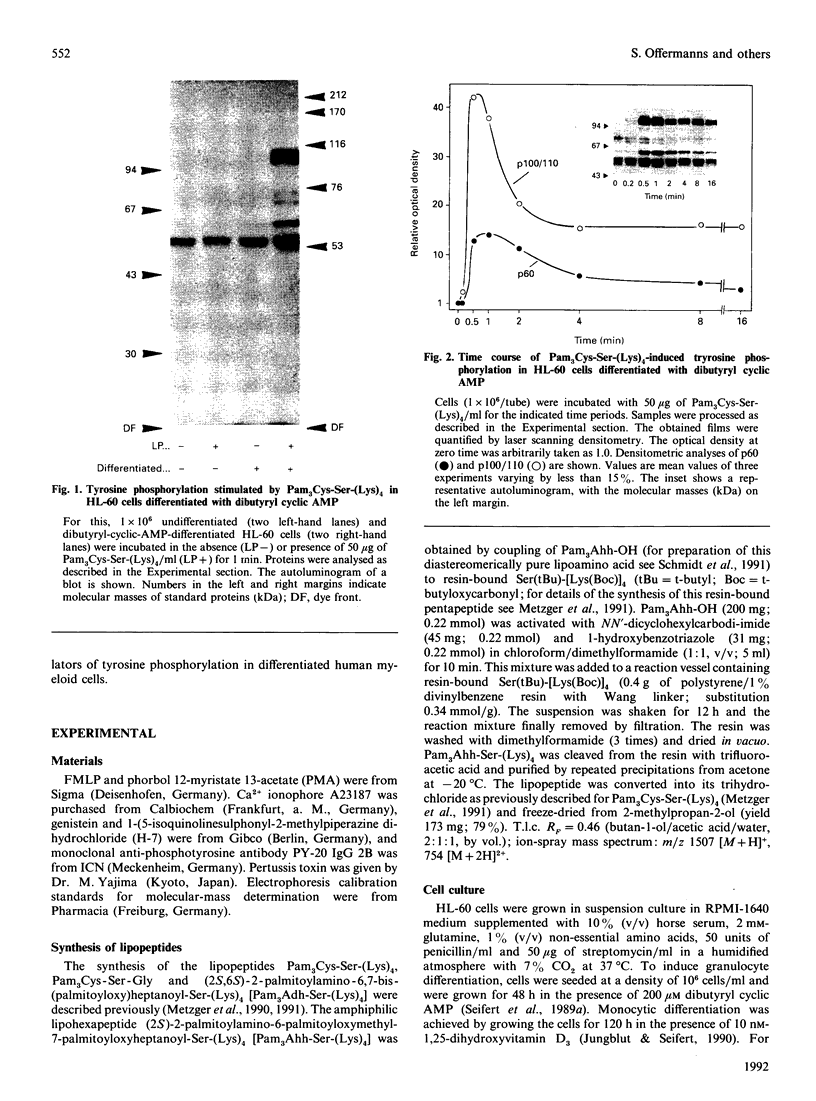
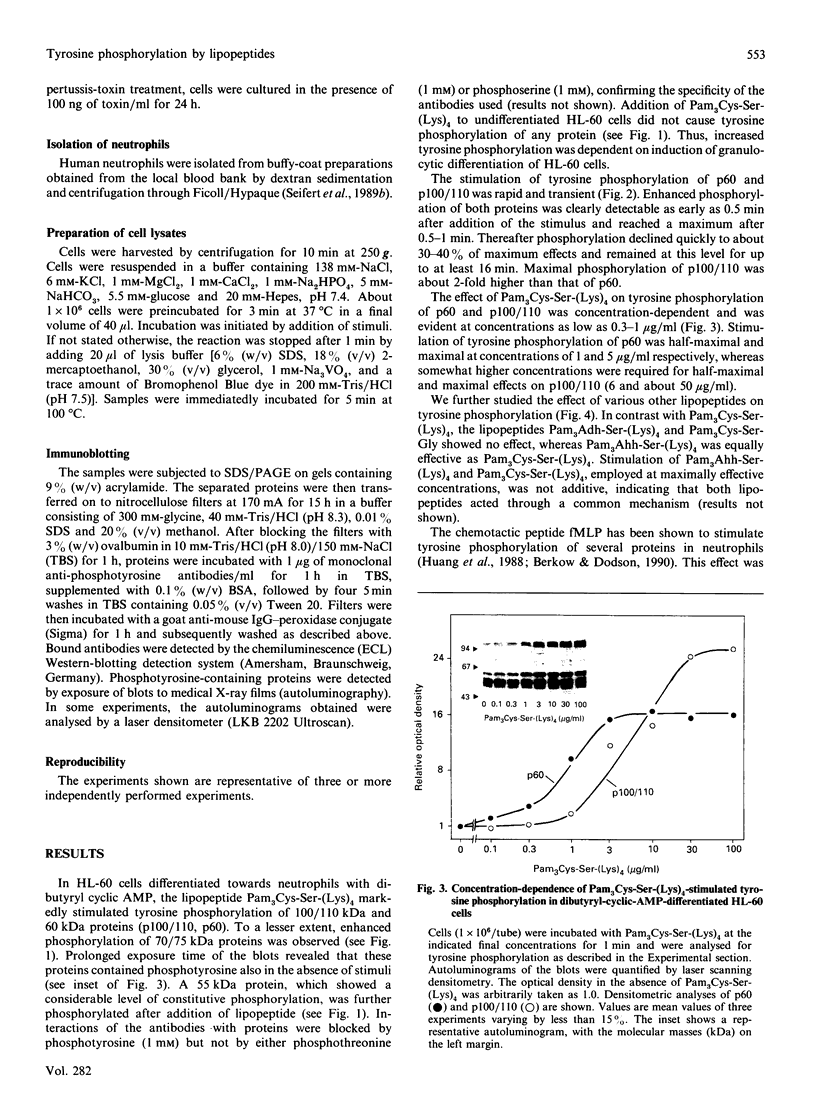
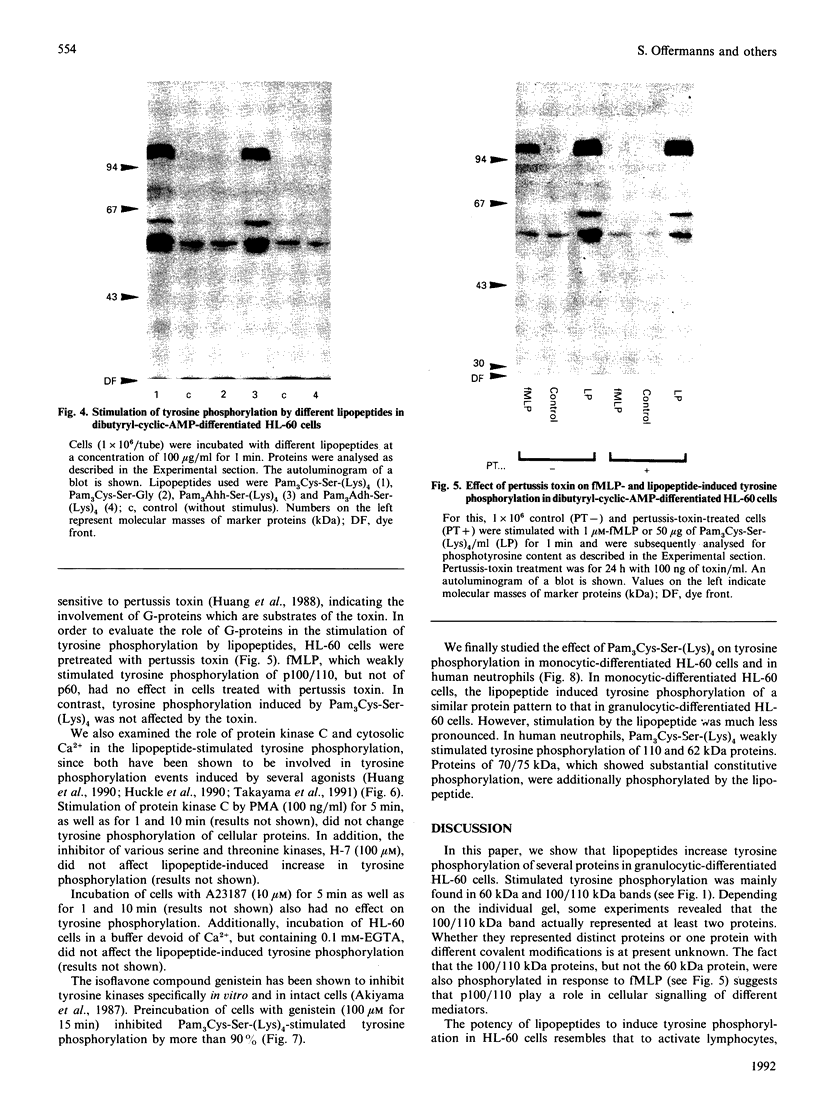
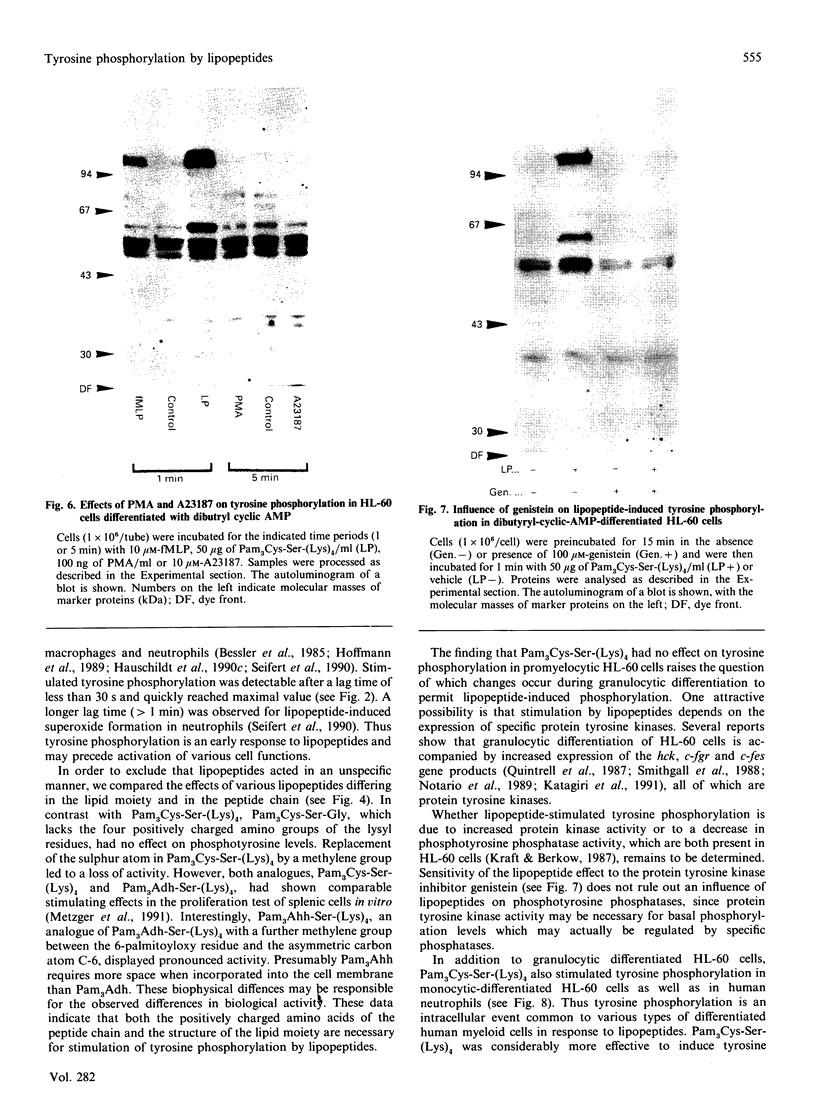
Synthetic lipopeptide analogues of the N-terminus of bacterial lipoprotein are effective activators of macrophages, neutrophils and lymphocytes. We studied the effect of the lipopeptide N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]- (R)-cysteinyl-(S)-seryl-(S)-lysyl-(S)-lysyl-(S)-lysyl-(S)-lysine [Pam3Cys-Ser-(Lys)4] on tyrosine phosphorylation in dibutyryl-cyclic-AMP-differentiated HL-60 cells, using anti-phosphotyrosine antibodies. Pam3Cys-Ser-(Lys)4 concentration-dependently stimulated tyrosine phosphorylation of 100/110 kDa and 60 kDa proteins and, to a lesser extent, of 55 kDa and 70/75 kDa proteins. Half-maximal and maximal effects were observed at concentrations of 1-6 and 5-50 micrograms/ml respectively. The lipopeptide-induced increase in phosphorylation was rapid and transient, with a peak response after 30-60 s. The lipopeptide (2S)-2-palmitoylamino-6-palmitoyloxymethyl-7-palmitoyloxy heptanoyl-Ser-(Lys)4 [Pam3Ahh-Ser-(Lys)4] was as potent as Pam3Cys-Ser(Lys)4, whereas (2S,6S)-2-palmitoylamino-6,7-bis(palmitoyloxy)heptanoyl++ +-Ser-(Lys)4 [Pam3Adh-Ser-(Lys)4] and Pam3Cys-Ser-Gly did not induce tyrosine phosphorylation. Lipopeptide-induced tyrosine phosphorylation was not affected by treatment of cells with pertussis toxin. Neither phorbol 12-myristate 13-acetate nor A23187 induced tyrosine phosphorylation in dibutyryl-cyclic-AMP-differentiated HL-60 cells. In HL-60 promyelocytes, Pam3Cys-Ser-(Lys)4 had no effect on tyrosine phosphorylation, whereas the lipopeptide also induced tyrosine phosphorylation in 1,25-dihydroxyvitamin-D3-differentiated HL-60 cells and in human neutrophils. These results show that lipopeptides are effective stimulators of tyrosine phosphorylation in mature human myeloid cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Berkow R. L., Dodson R. W., Kraft A. S. Human neutrophils contain distinct cytosolic and particulate tyrosine kinase activities: possible role in neutrophil activation. Biochim Biophys Acta. 1989 Aug 31;997(3):292–301. doi: 10.1016/0167-4838(89)90200-8. [DOI] [PubMed] [Google Scholar]
- Berkow R. L., Dodson R. W. Tyrosine-specific protein phosphorylation during activation of human neutrophils. Blood. 1990 Jun 15;75(12):2445–2452. [PubMed] [Google Scholar]
- Bessler W. G., Cox M., Lex A., Suhr B., Wiesmüller K. H., Jung G. Synthetic lipopeptide analogs of bacterial lipoprotein are potent polyclonal activators for murine B lymphocytes. J Immunol. 1985 Sep;135(3):1900–1905. [PubMed] [Google Scholar]
- Biesert L., Scheuer W., Bessler W. G. Interaction of mitogenic bacterial lipoprotein and a synthetic analogue with mouse lymphocytes. Isolation and characterization of binding proteins. Eur J Biochem. 1987 Feb 2;162(3):651–657. doi: 10.1111/j.1432-1033.1987.tb10687.x. [DOI] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Huang C. K., Bonak V. A., Wang E., Casnellie J. E., Shiraishi T., Sha'afi R. I. Tyrosine phosphorylation in human neutrophil. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1478–1485. doi: 10.1016/0006-291x(89)90841-3. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Tyrosine phosphorylation and oxygen consumption induced by G proteins in neutrophils. Am J Physiol. 1991 May;260(5 Pt 1):C1019–C1027. doi: 10.1152/ajpcell.1991.260.5.C1019. [DOI] [PubMed] [Google Scholar]
- Hauschildt S., Hoffmann P., Beuscher H. U., Dufhues G., Heinrich P., Wiesmüller K. H., Jung G., Bessler W. G. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: cytokine production, phagocytosis and Ia expression. Eur J Immunol. 1990 Jan;20(1):63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- Hauschildt S., Lückhoff A., Mülsch A., Kohler J., Bessler W., Busse R. Induction and activity of NO synthase in bone-marrow-derived macrophages are independent of Ca2+. Biochem J. 1990 Sep 1;270(2):351–356. doi: 10.1042/bj2700351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschildt S., Wolf B., Lückhoff A., Bessler W. G. Determination of second messengers and protein kinase C in bone marrow derived macrophages stimulated with a bacterial lipopeptide. Mol Immunol. 1990 Jun;27(6):473–479. doi: 10.1016/0161-5890(90)90065-8. [DOI] [PubMed] [Google Scholar]
- Hoffmann P., Wiesmüller K. H., Metzger J., Jung G., Bessler W. G. Induction of tumor cytotoxicity in murine bone marrow-derived macrophages by two synthetic lipopeptide analogues. Biol Chem Hoppe Seyler. 1989 Jun;370(6):575–582. doi: 10.1515/bchm3.1989.370.1.575. [DOI] [PubMed] [Google Scholar]
- Huang C. K., Bonak V., Laramee G. R., Casnellie J. E. Protein tyrosine phosphorylation in rabbit peritoneal neutrophils. Biochem J. 1990 Jul 15;269(2):431–436. doi: 10.1042/bj2690431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. K., Laramee G. R., Casnellie J. E. Chemotactic factor induced tyrosine phosphorylation of membrane associated proteins in rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1988 Mar 15;151(2):794–801. doi: 10.1016/s0006-291x(88)80351-6. [DOI] [PubMed] [Google Scholar]
- Huckle W. R., Prokop C. A., Dy R. C., Herman B., Earp S. Angiotensin II stimulates protein-tyrosine phosphorylation in a calcium-dependent manner. Mol Cell Biol. 1990 Dec;10(12):6290–6298. doi: 10.1128/mcb.10.12.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jungblut P. R., Seifert R. Analysis by high-resolution two-dimensional electrophoresis of differentiation-dependent alterations in cytosolic protein pattern of HL-60 leukemic cells. J Biochem Biophys Methods. 1990 Jun;21(1):47–58. doi: 10.1016/0165-022x(90)90044-d. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Berkow R. L. Tyrosine kinase and phosphotyrosine phosphatase activity in human promyelocytic leukemia cells and human polymorphonuclear leukocytes. Blood. 1987 Aug;70(2):356–362. [PubMed] [Google Scholar]
- Metzger J., Jung G., Bessler W. G., Hoffmann P., Strecker M., Lieberknecht A., Schmidt U. Lipopeptides containing 2-(palmitoylamino)-6,7-bis(palmitoyloxy) heptanoic acid: synthesis, stereospecific stimulation of B-lymphocytes and macrophages, and adjuvanticity in vivo and in vitro. J Med Chem. 1991 Jul;34(7):1969–1974. doi: 10.1021/jm00111a008. [DOI] [PubMed] [Google Scholar]
- Metzger J., Wiesmüller K. H., Schaude R., Bessler W. G., Jung G. Synthesis of novel immunologically active tripalmitoyl-S-glycerylcysteinyl lipopeptides as useful intermediates for immunogen preparations. Int J Pept Protein Res. 1991 Jan;37(1):46–57. doi: 10.1111/j.1399-3011.1991.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Nasmith P. E., Mills G. B., Grinstein S. Guanine nucleotides induce tyrosine phosphorylation and activation of the respiratory burst in neutrophils. Biochem J. 1989 Feb 1;257(3):893–897. doi: 10.1042/bj2570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notario V., Gutkind J. S., Imaizumi M., Katamine S., Robbins K. C. Expression of the fgr protooncogene product as a function of myelomonocytic cell maturation. J Cell Biol. 1989 Dec;109(6 Pt 1):3129–3136. doi: 10.1083/jcb.109.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Lebo R., Varmus H., Bishop J. M., Pettenati M. J., Le Beau M. M., Diaz M. O., Rowley J. D. Identification of a human gene (HCK) that encodes a protein-tyrosine kinase and is expressed in hemopoietic cells. Mol Cell Biol. 1987 Jun;7(6):2267–2275. doi: 10.1128/mcb.7.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R., Burde R., Schultz G. Lack of effect of opioid peptides, morphine and naloxone on superoxide formation in human neutrophils and HL-60 leukemic cells. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jul;340(1):101–106. doi: 10.1007/BF00169214. [DOI] [PubMed] [Google Scholar]
- Seifert R., Schultz G., Richter-Freund M., Metzger J., Wiesmüller K. H., Jung G., Bessler W. G., Hauschildt S. Activation of superoxide formation and lysozyme release in human neutrophils by the synthetic lipopeptide Pam3Cys-Ser-(Lys)4. Involvement of guanine-nucleotide-binding proteins and synergism with chemotactic peptides. Biochem J. 1990 May 1;267(3):795–802. doi: 10.1042/bj2670795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithgall T. E., Yu G., Glazer R. I. Identification of the differentiation-associated p93 tyrosine protein kinase of HL-60 leukemia cells as the product of the human c-fes locus and its expression in myelomonocytic cells. J Biol Chem. 1988 Oct 15;263(29):15050–15055. [PubMed] [Google Scholar]
- Steffens U., Bessler W., Hauschild S. B cell activation by synthetic lipopeptide analogues of bacterial lipoprotein bypassing phosphatidylinositol metabolism and proteinkinase C translocation. Mol Immunol. 1989 Sep;26(9):897–904. doi: 10.1016/0161-5890(89)90146-6. [DOI] [PubMed] [Google Scholar]
- Takayama H., Nakamura T., Yanagi S., Taniguchi T., Nakamura S., Yamamura H. Ionophore A23187-induced protein-tyrosine phosphorylation of human platelets: possible synergism between Ca2+ mobilization and protein kinase C activation. Biochem Biophys Res Commun. 1991 Jan 31;174(2):922–927. doi: 10.1016/0006-291x(91)91506-8. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Yamanashi Y., Inoue K., Katagiri T., Sukegawa J., Semba K., Yamamoto T. Allotment of protein-tyrosine kinases belonging to the src-family. Adv Second Messenger Phosphoprotein Res. 1990;24:284–289. [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wolf B., Uhl B., Hauschildt S., Metzger J., Jung G., Bessler W. G. Interaction of the lymphoid cell line BCL1 with lipopeptide analogues of bacterial lipoprotein: electron energy loss spectroscopy (EELS) as a novel method to detect the distribution of the activator within the cells. Immunobiology. 1989 Nov;180(1):93–100. doi: 10.1016/S0171-2985(89)80033-6. [DOI] [PubMed] [Google Scholar]