Abstract
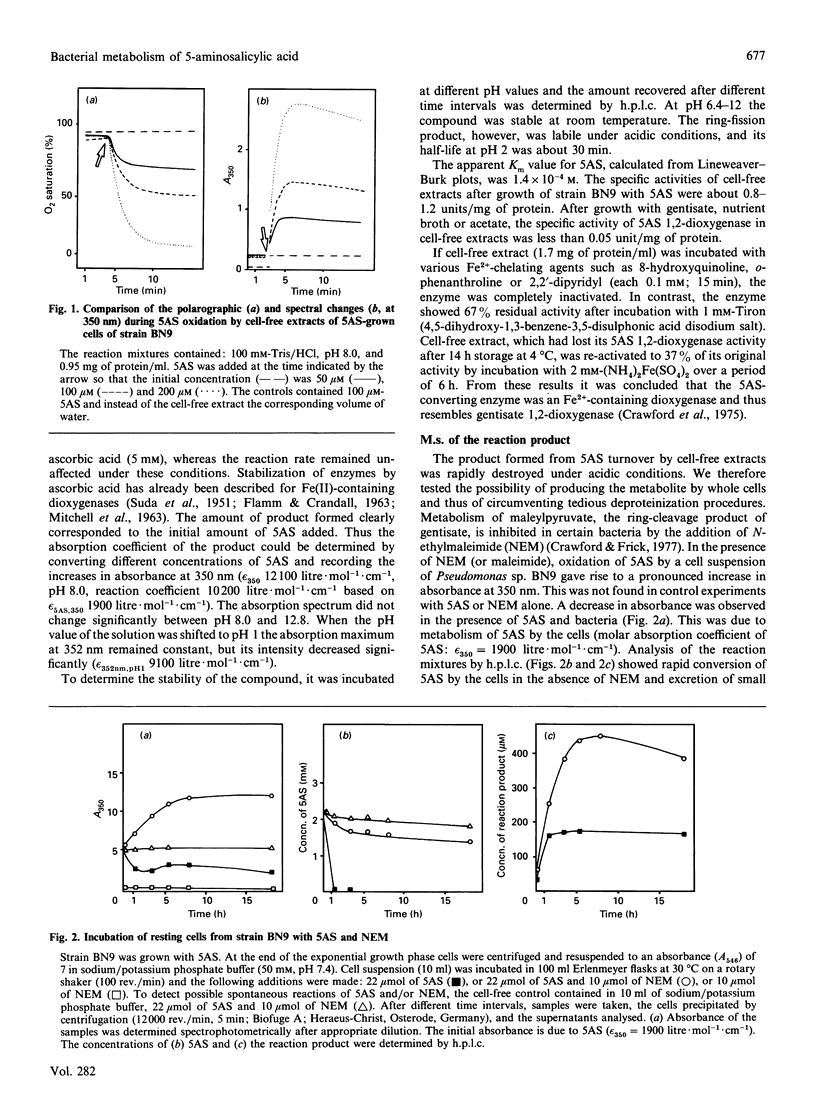
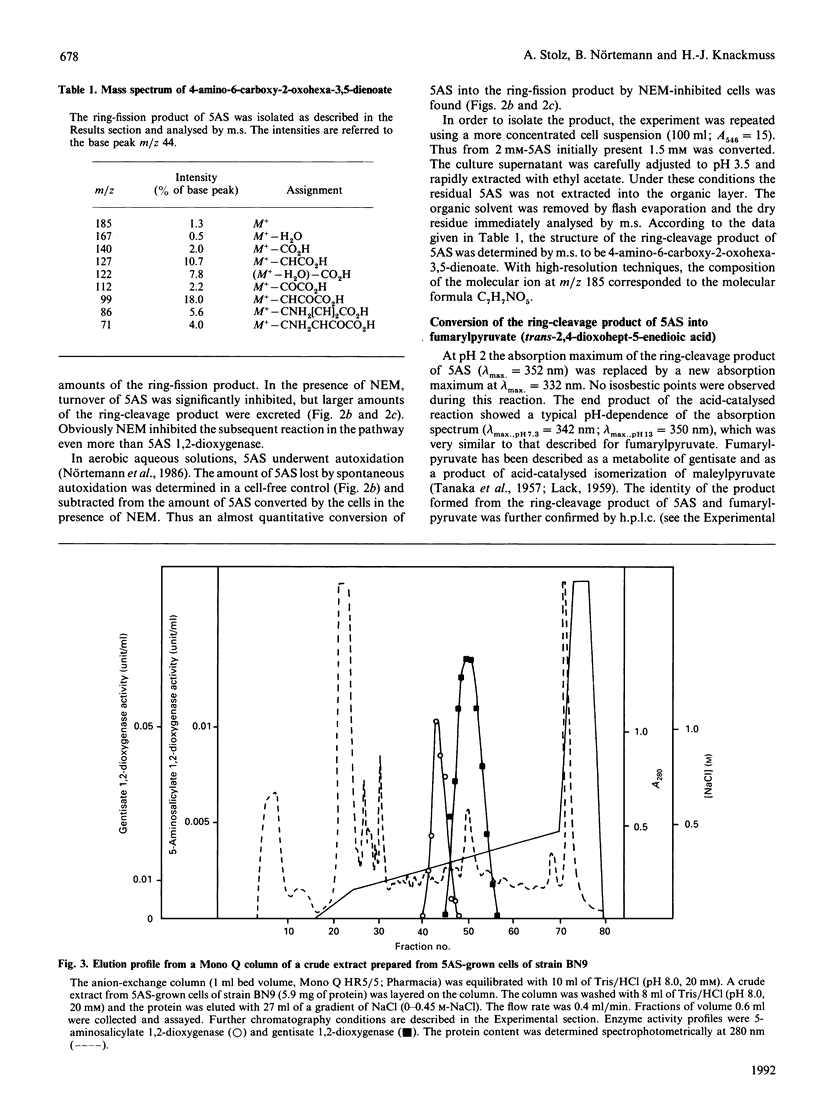
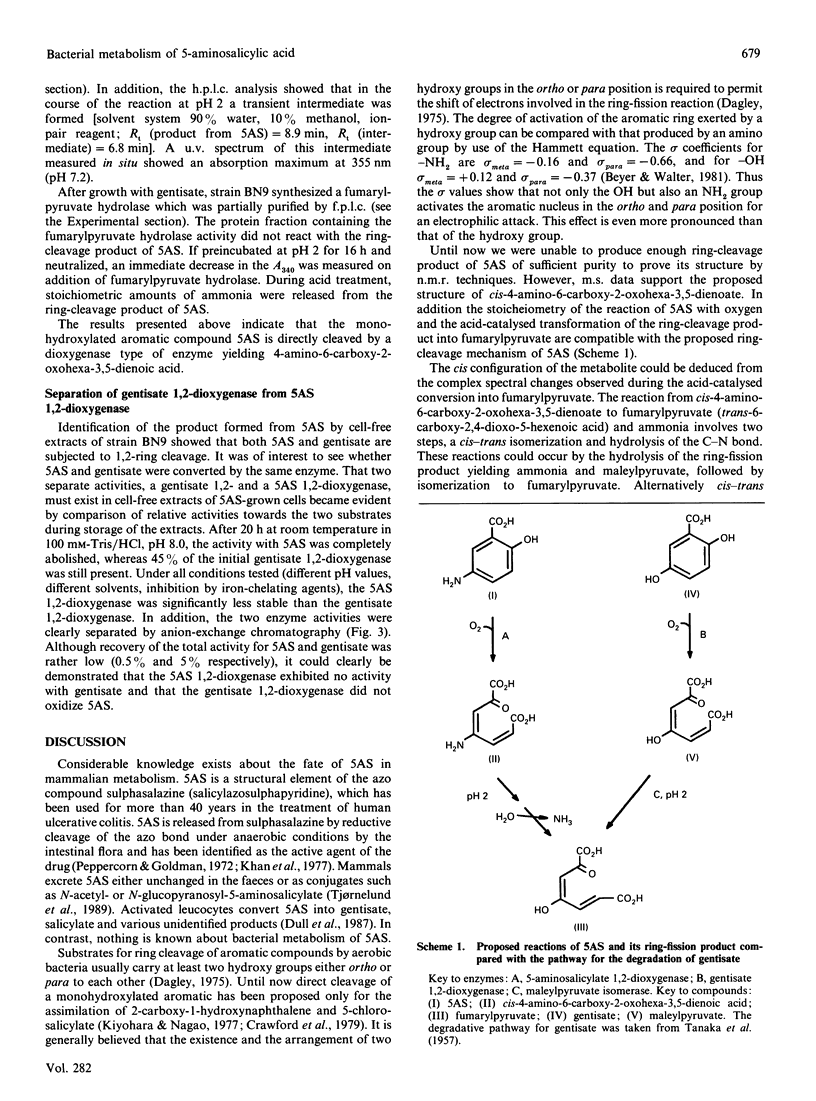
The metabolism of 5-aminosalicylate (5AS) by a bacterial strain, Pseudomonas sp. BN9, was studied. Intact cells of Pseudomonas sp. BN9 grown with 5AS oxidized 5AS and 2,5-dihydroxybenzoate (gentisate), whereas cells grown with gentisate oxidized only the growth substrate of all substituted salicylates tested. Cell extracts from Pseudomonas sp. BN9 catalysed the stoichiometric reaction of 1 mol of oxygen with 1 mol of 5AS to a metabolite with an intense u.v.-absorption maximum at 352 nm (pH 8.0). This metabolite was accumulated under neutral conditions, but was rapidly destroyed at acid pH. It was identified by m.s. and acid-catalysed deamination to fumarylpyruvate (trans-2,4-dioxohept-5-enedioic acid) as cis-4-amino-6-carboxy-2-oxohexa-3,5-dienoate, thus demonstrating direct cleavage of the monohydroxylated substrate 5AS to a non-aromatic ring-fission product. The enzyme responsible for conversion of 5AS was shown to be Fe(II)-dependent and to be distinct from gentisate 1,2-dioxygenase in strain BN9.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azad Khan A. K., Piris J., Truelove S. C. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet. 1977 Oct 29;2(8044):892–895. doi: 10.1016/s0140-6736(77)90831-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Crawford R. L., Frick T. D. Rapid spectrophotometric differentiation between glutathione-dependent and glutathione-independent gentisate and homogentisate pathways. Appl Environ Microbiol. 1977 Aug;34(2):170–174. doi: 10.1128/aem.34.2.170-174.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Hutton S. W., Chapman P. J. Purification and properties of gentisate 1,2-dioxygenase from Moraxella osloensis. J Bacteriol. 1975 Mar;121(3):794–799. doi: 10.1128/jb.121.3.794-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Olson P. E., Frick T. D. Catabolism of 5-chlorosalicylate by a Bacillus isolated from the Mississippi River. Appl Environ Microbiol. 1979 Sep;38(3):379–384. doi: 10.1128/aem.38.3.379-384.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECKER R. H., KANG H. H., LEACH F. R., HENDERSON L. M. Purification and properties of 3-hydroxyanthranilic acid oxidase. J Biol Chem. 1961 Nov;236:3076–3082. [PubMed] [Google Scholar]
- Dagley S. A biochemical approach to some problems of environmental pollution. Essays Biochem. 1975;11:81–138. [PubMed] [Google Scholar]
- Dull B. J., Salata K., Van Langenhove A., Goldman P. 5-Aminosalicylate: oxidation by activated leukocytes and protection of cultured cells from oxidative damage. Biochem Pharmacol. 1987 Aug 1;36(15):2467–2472. doi: 10.1016/0006-2952(87)90518-1. [DOI] [PubMed] [Google Scholar]
- FLAMM W. G., CRANDALL D. I. Purification of mammalian homogentisate oxidase and evidence for the existence of ferrous mercaptans in the active center. J Biol Chem. 1963 Jan;238:389–396. [PubMed] [Google Scholar]
- Foster J. W., Moat A. G. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol Rev. 1980 Mar;44(1):83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn S. R., Bradley G., Chapman P. J. Glutathione-independent isomerization of maleylpyruvate by Bacillus megaterium and other gram-positive bacteria. J Bacteriol. 1985 Aug;163(2):640–647. doi: 10.1128/jb.163.2.640-647.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter M. C., Patek D. R., Schellenberg K. A. Catalysis of -iminoglutarate formation from -ketoglutarate and ammonia by bovine glutamate dehydrogenase. J Biol Chem. 1972 Oct 10;247(19):6271–6276. [PubMed] [Google Scholar]
- Koontz W. A., Shiman R. Beef kidney 3-hydroxyanthranilic acid oxygenase. Purification, characterization, and analysis of the assay. J Biol Chem. 1976 Jan 25;251(2):368–377. [PubMed] [Google Scholar]
- Kuhm A. E., Schlömann M., Knackmuss H. J., Pieper D. H. Purification and characterization of dichloromuconate cycloisomerase from Alcaligenes eutrophus JMP 134. Biochem J. 1990 Mar 15;266(3):877–883. [PMC free article] [PubMed] [Google Scholar]
- LACK L. The enzymic oxidation of gentisic acid. Biochim Biophys Acta. 1959 Jul;34:117–123. doi: 10.1016/0006-3002(59)90239-2. [DOI] [PubMed] [Google Scholar]
- LADD J. N. Oxidation of anthranilic acid by a species of Achromobacter isolated from soil. Nature. 1962 Jun 16;194:1099–1100. doi: 10.1038/1941099b0. [DOI] [PubMed] [Google Scholar]
- Marcotte P., Walsh C. Sequence of reactions which follows enzymatic oxidation of propargylglycine. Biochemistry. 1978 Dec 26;17(26):5613–5619. doi: 10.1021/bi00619a005. [DOI] [PubMed] [Google Scholar]
- Nörtemann B., Baumgarten J., Rast H. G., Knackmuss H. J. Bacterial communities degrading amino- and hydroxynaphthalene-2-sulfonates. Appl Environ Microbiol. 1986 Nov;52(5):1195–1202. doi: 10.1128/aem.52.5.1195-1202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppercorn M. A., Goldman P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J Pharmacol Exp Ther. 1972 Jun;181(3):555–562. [PubMed] [Google Scholar]
- Porter D. J., Bright H. J. The kinetics of imino acid accumulation in the D-amino acid oxidase reaction. Biochem Biophys Res Commun. 1972 Jan 31;46(2):571–577. doi: 10.1016/s0006-291x(72)80177-3. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., HAYAISHI O. The bacterial oxidation of tryptophan; a study in comparative biochemistry. Science. 1951 Sep 28;114(2961):326–330. doi: 10.1126/science.114.2961.326. [DOI] [PubMed] [Google Scholar]
- Tjørnelund J., Hansen S. H., Cornett C. New metabolites of the drug 5-aminosalicylic acid. I: N-beta-D-glucopyranosyl-5-aminosalicylic acid. Xenobiotica. 1989 Aug;19(8):891–899. doi: 10.3109/00498258909043149. [DOI] [PubMed] [Google Scholar]
- VESCIA A., DI PRISCO G. Studies on purified 3-hydroxyanthranilic acid oxidase. J Biol Chem. 1962 Jul;237:2318–2324. [PubMed] [Google Scholar]
- Wheelis M. L., Palleroni N. J., Stanier R. Y. The metabolism of aromatic acids by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol. 1967;59(1):302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]


