Abstract
Background and Aims
Multiple developments of squamous dysplasia and squamous cell carcinoma (SCC) in the upper aerodigestive tract have been explained by field cancerization phenomenon and were associated with alcohol and cigarette use. Second primary SCC development after curative treatment impairs patients’ quality of life and survival; however, how these consumption and cessation affect field cancerization is still unknown.
Methods
This is a multicenter cohort study including 331 patients with superficial esophageal SCC (ESCC) treated endoscopically and pooled data from 1022 healthy subjects for comparison. Physiological condition in the background esophageal mucosa was classified into 3 groups based on the number of Lugol-voiding lesions (LVLs) per endoscopic view: grade A, 0; grade B, 1–9; or grade C, ≥10 LVLs. Lifestyle surveys were conducted using a self-administered questionnaire. Patients were counseled on the need for alcohol and smoking cessation by physicians and were endoscopically surveyed every 6 months.
Results
LVL grades were positively associated with alcohol drinking intensity, flushing reactions, smoking, and high-temperature food and were negatively associated with eating green and yellow vegetables and fruit. Second primary ESCC and head/neck SCC were significantly more prevalent in the grade C LVL (cumulative 5-y incidences 47.1%, 95% confidence interval [CI] = 38.0–57.2 and 13.3%, 95% CI = 8.1–21.5, respectively). Alcohol and smoking cessation significantly reduced the development of second primary ESCC (adjusted hazard ratios 0.47, 95% = CI 0.26–0.85 and 0.49, 95% CI = 0.26–0.91, respectively).
Conclusion
Alcohol drinking, smoking, flushing reaction, and high-temperature food were closely associated with field cancerization, and cessation of alcohol and smoking significantly reduced the risk of development of second primary cancer. UMIN Clinical Trials Registry ID:UMIN000001676.
Keywords: Field Cancerization, Risk Reduction, Esophageal Cancer, Cessation of Alcohol Drinking, Cessation of Cigarette Smoking
Graphical abstract
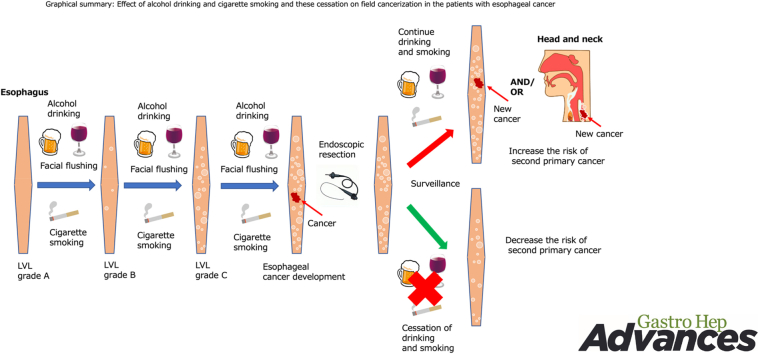
Alcohol and cigarette use are established risk factors for various cancers.1,2 In particular, they are definite carcinogens for esophageal squamous cell carcinoma (ESCC) and head and neck SCCs (HNSCCs), and this combination shows robust synergic interactions for the risk.3, 4, 5, 6 In addition, SCC and squamous dysplastic epithelium develop multifocally in these organs. This phenomenon has been explained by the “field cancerization” and is closely associated with alcohol and cigarette use.7 However, it has been unclear how multiple dysplastic epithelium would interact with other factors.
The field cancerization phenomenon impairs patients’ quality of life and survival because they are at risk of multiple cancers even after curative treatment.8 Although cessation of alcohol and cigarette use could be an ideal strategy for reducing the risk of field cancerization, the effect of this cessation on development of squamous dysplastic epithelium and second primary cancer has been unclear.
We previously reported that physiologically normal esophageal epithelium expands aging-related driver-mutated cancer clones, depending on alcohol and cigarette use, and can affect field cancerization.9 Squamous dysplastic epithelium has been recognized as a precancerous lesion; however, it is difficult to clinically identify. Lugol chromoendoscopy can clinically visualize this lesion as the Lugol-voiding lesion (LVL).10 Multiple LVLs are closely associated with inactive aldehyde dehydrogenase 2 (ALDH2) and field cancerization.8,10, 11, 12, 13 ALDH2 is a key enzyme to detoxify acetaldehyde, the first metabolite of ethanol and a definite carcinogen for ESCC and HNSCC.10, 11, 12, 13, 14 The subject with inactive ALDH2 shows acetaldehyde-induced flushing reaction. In addition, acetaldehyde is a major carcinogenic compound of cigarette smoke.14,15 The acetaldehyde concentrations used far exceeded those reached in the saliva by humans while smoking.16,17 However, cigarette smoke–derived acetaldehyde is not absorbed into circulation. Although cigarette smoke–derived acetaldehyde might affect directly the epithelium of the head and neck region and the esophagus, the effects of combination between acetaldehyde-induced flushing reaction (phenotypic markers of the ALDH2 genotype), alcohol drinking intensity, smoking, and other risk factors on development of multiple dysplastic epithelium in these regions are not clearly understood.
Patients with superficial ESCC can be treated curatively by endoscopic resection if they have no lymph node metastases. In this way, they can preserve the organ and have prolonged survival. Conversely, they are at risk of multiple cancers in the preserved organ via field cancerization. Investigating the continuous effects of alcohol and cigarette use and their cessation on field cancerization among these patients will help shed light on the research question. Therefore, we conducted a Japan esophageal cohort study and have already reported that multiple LVLs in the esophagus increased the risks of second primary ESCC and HNSCC and that cessation of alcohol reduced the risk of a second primary ESCC.13 However, the previous report could not show the comparison with cancer-free healthy subjects. Furthermore, long-term effect of cessation of alcohol and cigarette use on field cancerization is still unclear because the previous report had a short follow-up period.
In this study, we initially investigated whether alcohol intake and abstinence affect acetaldehyde-induced DNA damage on the esophageal epithelium in individuals with ALDH2 dysfunction using a mouse model. In addition, we investigated whether direct acetaldehyde exposure on esophageal epithelial cells induces gene mutations. Next, we showed the following research questions: how the various risk factors affect development of multiple dysplastic epithelia in the esophagus comparing between cancer-free healthy subjects and patients with ESCC and how long-term cessation of alcohol and cigarette use affects field cancerization.
Methods
In Vivo and in Vitro Study
To investigate the effects of alcohol intake on acetaldehyde-mediated DNA damage in individuals with ALDH2 dysfunction, ALDH2 knockout (KO) (−/−) mice,18 heterozygous (+/−) mice, and wild-type (WT) (+/+) mice (C57BL/6N male mice) were allowed to drink 10% ethanol freely for 14 days and esophageal tissues were collected on days 0, 1, 3, 7, and 14 after the start of 10% ethanol drinking (n = 3, each group). DNA was extracted form esophageal tissues, and acetaldehyde-derived DNA adduct levels were evaluated by measuring N2-ethylidene-2′-deoxyguanosine (N2-ethylidene-dG) levels. Next, to investigate whether esophageal N2-ethylidene-dG levels in mice with ALDH2 dysfunction decrease after alcohol cessation, ALDH2 KO mice and/or WT mice were allowed to drink 10% ethanol for 7 days, and thereafter, they drank ethanol-free water for 14 days. We collected the esophagus and extracted DNA samples on days 0, 3, 7, 10, 14, and 21 after the start of 10% ethanol drinking, and esophageal N2-ethylidene-dG levels were measured (n = 4, each group).
Mice were euthanized painlessly under anesthesia with diethyl ether inhalation followed by cervical dislocation. Esophageal tissues were collected in 4% paraformaldehyde phosphate buffer solution (Wako Pure Chemical Industries, Ltd, Osaka, Japan) and frozen in liquid nitrogen and stored at −80 °C for analysis of DNA adducts. N2-ethylidene-dG levels in esophageal tissue were measured by liquid chromatography tandem mass spectrometry as reported.19 All experiments conformed to the relevant regulatory standards and were approved by the institutional animal care and use committee of Kyoto University (Med Kyo 16196).
To confirm whether acetaldehyde-induced genetic abnormalities occur, we exposed immortalized human esophageal epithelial cells (EPC2-hTERT)20 to acetaldehyde for 2 months, cloned single cells, and performed whole genome sequencing (n = 3, each group). Whole genome sequencing was conducted as previously reported.9 For comparison with the COSMIC signatures, mutations were allocated to 30 COSMIC mutational patterns using R package Mutational Patterns.21
DNA Adduct Level in Human Esophagus
To investigate whether acetaldehyde-mediated DNA damage could be detected in the human esophageal mucosa, we measured N2-ethylidene-dG levels in the esophageal mucosa in 11 healthy subjects (ALDH2 WT: n = 5, ALDH2 inactive type: n = 6). In addition, we compared them with amount of alcohol intake. Furthermore, we measured N2-ethylidene-dG levels in the background non-neoplastic esophageal mucosa of 10 patients with ESCC and examined the relationship between LVL grade and N2-ethylidene-dG levels. This study was approved by the ethics committee of Kyoto University (G341 and G1067).
Cohort Study Design and Participants
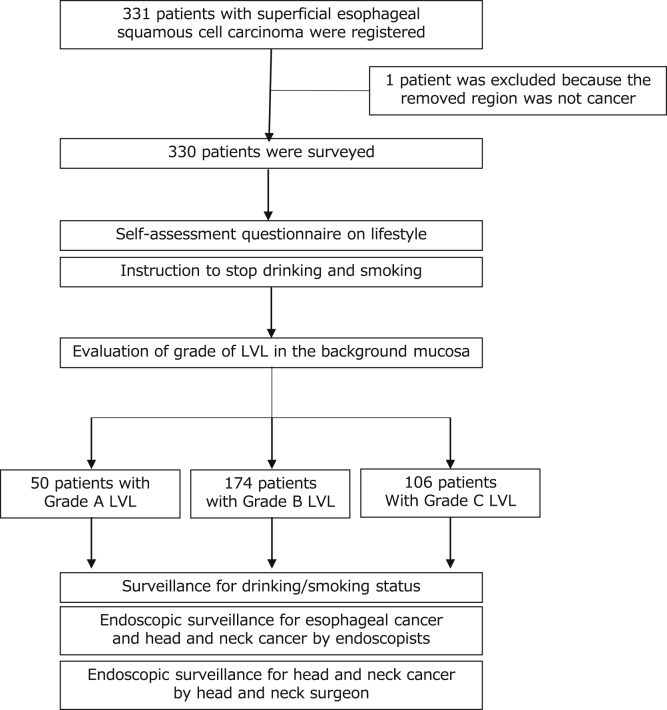
This cohort study analyzed 331 patients with newly diagnosed superficial ESCC from 16 hospitals in Japan.13 All tumors were removed by endoscopic resection. This study was approved by the institutional review board at each hospital, and all participants provided written informed consent. The eligibility criteria have been reported.13 Registration and the surveillance schedule are shown in Figure 1. This study conforms to the STROBE guidelines.
Figure 1.
Registration and surveillance flow of the JEC study. JEC, Japan esophageal cohort.
Different cross-sectional data resources (n = 1022) in our previous study were used as a historical healthy control group.11,12 These cancer-free healthy subjects consisted of 610 men and 412 women, aged 40–79 years. They were ordinary residents or workers living in Tokyo or neighboring areas who underwent annual routine health checkups including endoscopy.
All authors had access to the study data and reviewed and approved the final manuscript.
Evaluation of Squamous Dysplastic Epithelium
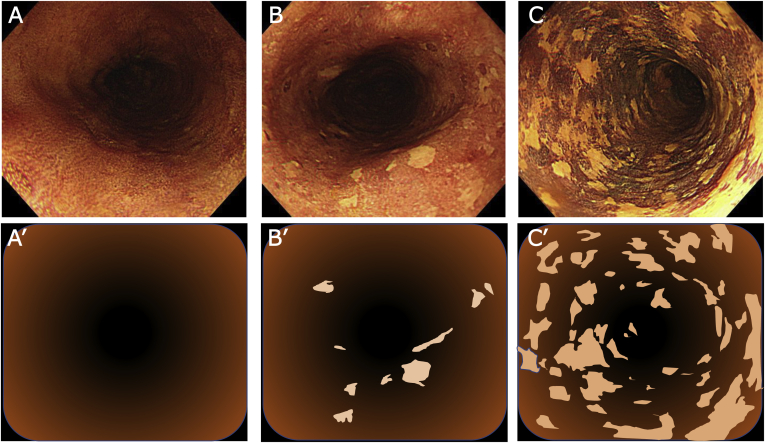
Histological evaluation for each squamous dysplastic epithelium in the entire esophageal mucosa is clinically impossible. Therefore, we used Lugol chromoendoscopy to identify the squamous dysplastic lesion as an LVL even for endoscopically normal mucosa. As reported previously,13 patients with ESCC were classified into 3 groups as per the LVL grade: grade A, no LVL per endoscopic view; grade B, 1–9 LVLs per endoscopic view; and grade C, ≥10 LVLs per endoscopic view (Figure 2). All the images were reviewed and were sorted into these grades by central review board of 3 certified endoscopists. Lugol chromoendoscopy was intensive examination and causes mucosal irritation, chest pain, discomfort, and sometimes allergic shock to iodine solution. Then, it is not suitable for annual routine checkup and so we did not indicate this examination for cancer-free healthy subjects. Therefore, we categorized the data from all cancer-free subjects as a reference.
Figure 2.
Grade of Lugol-voiding lesions (LVLs). The numbers of LVLs per endoscopic view were counted and divided into 3 categories: A and A’: group A, no lesions; B and B’: group B, 1–9 lesions, C and C’: group C, 10 or more lesions.
Questionnaire
Lifestyle surveys were conducted using a self-administered questionnaire at study entry. Histories of drinking, alcohol flushing response, smoking, and consumption of high-temperature food, green-yellow vegetables, and fruit were documented carefully as described previously.13 Drinking status was categorized into 5 groups: never/rarely, <1 unit/wk; light, 1–8.9 units/wk; moderate, 9–17.9 units/wk; heavy, ≥18 units/wk; and ex-drinker (1 unit = 22 g ethanol). Smoking status was categorized into 3 groups: never, 0 pack-years; light, <30 pack-years; and heavy, ≥30 pack-years. The same questionnaire was used to obtain detailed information on each subject including healthy controls. Any records of facial flushing were assumed to be indicative of inactive ALDH2, and the absence of flushing was assumed to be indicative of active ALDH2.22
Education About Cessation of Alcohol and Cigarette Use
At study entry, the physicians in charge handed out a document describing the importance of alcohol and smoking cessation to all patients and verbally advised them to stop drinking and smoking. At each surveillance examination, we checked on their drinking status (drinking ceased or continuing; for drinkers, the drinking frequency and amount of alcohol consumed) and smoking status (smoking ceased or continuing; for smokers, the number of cigarettes smoked per day) by self-reporting and attempted to reeducate them not to drink or smoke.
Definition of Cessation of Alcohol and Cigarette Use
If the patients continued cessation of alcohol drinking and/or cigarette smoking at the final surveillance examination by self-reporting or at the time of developing a second primary ESCC or HNSCC, they were classified as the cessation group. If the patients continued drinking and/or smoking, they were classified as the noncessation group. Patients who had already quit drinking or smoking at the time of entry were not included in the cessation or noncessation groups.
Endoscopic Surveillance and Definition of Second Primary Cancer
Endoscopic surveillance of the head and neck region and esophagus was performed at 3-month intervals for up to 6 months after endoscopic resection. Subsequently, these surveillances were repeated every 6 months. The head and neck regions were examined using laryngoscopy by an otolaryngologist at the time of entry and at 1-year intervals thereafter. In addition to otolaryngologists, gastrointestinal endoscopists also examined the oropharynx and hypopharynx during the surveillance endoscopies. If a new lesion in the esophagus or head and neck region was detected and was confirmed histologically to be SCC, such a lesion was defined as a second primary ESCC or HNSCC. Local recurrence at the primary site was not included as a new lesion.
Outcome Measure
The primary outcome of this study measures the risk factors for development of multiple LVLs in the esophagus. Secondary outcome measures accumulated incidence of second primary ESCC and HNSCC after cessation of alcohol and smoking as per the grade of the LVL.
Statistical Analysis
Percentage values were adjusted for sex and age by direct methods using all cancer cases as the standard population and evaluated statistically using the Cochran–Mantel–Haenszel test. Mean values were adjusted for sex and age and tested statistically using analysis of covariance. Multiple logistic regression analysis was used to estimate the sex- and age-adjusted odds ratio (OR) and 95% confidence interval (CI). The Kaplan–Meier method and log–rank test were performed for the analysis of the development of second primary ESCC and HNSCC. We defined the time to development of these from the initial day of endoscopic treatment to the day of endoscopic diagnosis of a second primary tumor. A Cox proportional-hazards model was used to estimate the sex- and age-adjusted hazard ratio (HR) and 95% CI of the event. The analysis was conducted after the completion of at least 5 years of follow-up observations for all patients from the time of enrollment. Data for patients in whom follow-up examinations were not completed were censored at the last observation. All data were analyzed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). Statistical tests were 2-sided, and P values of <.05 were considered to indicate statistical significance.
Results
In Vivo and in Vitro Study
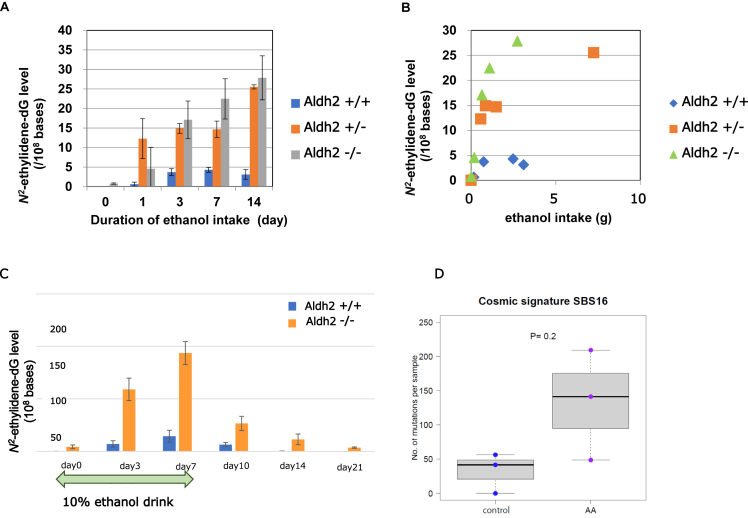
In mice, acetaldehyde-derived DNA adduct (N2-ethylidene-dG) was accumulated over time in the esophagus of mice with 10% ethanol ingestion. As shown in Figure 3A, esophageal DNA adduct levels in ALDH2+/− and/or ALDH2−/− mice ingested with 10% alcohol were significantly higher than those in ALDH2+/+ mice with alcohol ingestion at each collection date (days 3, 7, and 14). In addition, the N2-ethylidene-dG level was higher in ALDH2-deficient mice than that in ALDH2 WT mice even after ingestion of the same amount of ethanol and was higher in homozygous KO mice than that in heterozygous KO mice (Figure 3B).
Figure 3.
In vitro and in vivo study for acetaldehyde-derived DNA damages. Panel A showed time course of esophageal DNA adduct (N2-ethylidene-dG) levels in ALDH2 wild-type (ALDH2+/+), ALDH2 heterozygous (ALDH2+/−), and homozygous knockout (ALDH2−/−) mice with 10% ethanol for the indicated time durations. Panel B showed association of ethanol-intake volume and esophageal DNA adduct (N2-ethylidene-dG) level in ALDH2 wild-type (ALDH2+/+), ALDH2 heterozygous (ALDH2+/−), and homozygous knockout (Aldh2−/−) mice. Panel C showed time course of DNA adduct (N2-ethylidene-dG) in the esophagus during ethanol intake and after cessation of ethanol among Aldh2 wild-type (ALDH2+/+) and ALDH2 homozygous knockout (ALDH2−/−) mice. Panel D showed mutational signature of immortalized human esophageal epithelial cells (EPC2-hTERT) which were exposed to acetaldehyde (0.2mM) for 2 months.
After cessation of 10% ethanol intake for 7 days in ALDH2−/− and ALDH2+/+ mice, the N2-ethylidene-dG level in the esophagus has decreased, but it was still detectable on day 14 in ALDH2−/− mice, whereas it was below the detection limit on day 7 after cessation in ALDH2+/+ mice (Figure 3C).
Whole genome sequencing for esophageal epithelial cells showed that alcohol-related signature (COSMIC signature SBS16) was induced more in the acetaldehyde-exposed group (AA) than that in the control group (Figure 3D). The number of samples was too small to detect a significant difference.
DNA Adduct Level in the Human Esophagus
The esophageal N2-ethylidene-dG level in healthy subjects showed a good positive correlation (R2 = 0.9584, P < .001) with alcohol consumption, but it was not affected by the ALDH2 genotype (Figure A1). In the patients with ESCC, although we could not detect N2-ethylidene-dG in the esophageal mucosa of the grade A LVL, we could detect N2-ethylidene-dG in some mucosa of the grade C LVL (Table A1). Of note, N2-ethylidene-dG was not detected even in the esophageal mucosa of patients with ESCC who quitted drinking and smoking after diagnosis of ESCC. In contrast, a high esophageal N2-ethylidene-dG level was detected in subjects with a grade C LVL who were unknown for abstinence from alcohol and smoking (Table A1).
Participant Characteristics
Among the 331 patients, one was excluded because the lesion was not diagnosed definitively SCC as described previously.13 Table summarizes the selected demographic and clinical characteristics of the study population. Compared with the healthy control group, the proportion of men was higher in the more severe LVL grade groups (59.7% in the controls vs 62.0% in grade A, 85.1% in grade B, and 93.4% in grade C; P < .0001 vs controls). The proportion of subjects aged ≥70 years was higher in all LVL grade groups than controls (6.1% in controls, vs 42.0% in grade A, 46.0% in grade B, and 24.5% in grade C, P < .0001 vs controls). After adjusting for sex and age, the LVL grade was associated with progressively higher proportions of heavy drinkers (8.3% in controls vs 27.7% in grade A, 26.2% in grade B, and 52.8% in grade C; P < .0001 for trend), heavy smokers (34.4% in controls, vs 41.1% in grade A, 65.9% in grade B, and 71.0 % in grade C; P < .0001 for trend), high-temperature food (4.7% in controls, vs 18.4% in grade A, 20.8% in grade B, and 20.6 % in grade C; P < .0001 for trend), and alcohol-related flushing (54.8% in controls, vs 64.8% in grade A, 68.5% in grade B, and 59.4% in grade C; P < .0001 for trend). Eating green-yellow vegetables and fruit almost every day was negatively associated with the LVL grade (44.7% in controls; vs 48.0% in grade A, 45.1% in grade B, and 28.7% in grade C; P < .0001 for trend; 48.5% in control, 24.1% in grade A, 32.0% in grade B, and 24.5% in grade C, respectively; P < .0001 for trend).
Table.
Comparison of Clinical Characteristics Between Controls and Patients Within Each LVL Group
| Factor | Controls |
Grade of LVL |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
P | ||||||
| (n = 1022) | (n = 50) | (n = 174) | (n = 106) | ||||||
| Sex | |||||||||
| Men—n (%) | 610 | (59.7) | 31 | (62.0) | 148 | (85.1) | 99 | (93.4) | |
| Women—n (%) | 412 | (40.3) | 19 | (38.0) | 26 | (14.9) | 7 | (6.6) | <.0001 |
| P vs controls | – | .77 | <.0001 | <.0001 | |||||
| Age (y)—n (%) | |||||||||
| 40–59 | 560 | (54.8) | 11 | (22.0) | 30 | (17.2) | 21 | (19.8) | |
| 60–69 | 400 | (39.1) | 18 | (36.0) | 64 | (36.8) | 59 | (55.7) | |
| 70+ | 62 | (6.1) | 21 | (42.0) | 80 | (46.0) | 26 | (24.5) | <.0001 |
| P vs controls | – | <.0001 | <.0001 | <.0001 | |||||
| Mean (SD) | 58.8 | (7.3) | 66.4 | (9.9) | 67.7 | (8.6) | 64.8 | (6.5) | <.0001 |
| P vs controls | – | <.0001 | <.0001 | <.0001 | |||||
| Sex–age-adjusted % or mean | |||||||||
| Alcohol drinkinga,b—n (%) | |||||||||
| Never/rare | 397 | (38.6) | 19 | (25.9) | 14 | (7.9) | 2 | (4.7) | |
| Light | 300 | (30.3) | 7 | (13.8) | 40 | (21.4) | 8 | (8.9) | |
| Moderate | 192 | (21.3) | 11 | (27.8) | 51 | (30.9) | 28 | (25.5) | |
| Heavy | 110 | (8.3) | 10 | (27.7) | 44 | (26.2) | 62 | (52.8) | |
| Ex-drinker | 23 | (1.4) | 3 | (4.7) | 25 | (13.6) | 6 | (8.2) | <.0001 |
| P vs controls | – | .0006 | <.0001 | <.0001 | |||||
| Mean (SE) | 5.9 | (0.4) | 9.5 | (1.5) | 13.9 | (0.8) | 20.4 | (1.0) | <.0001 |
| P vs controls | – | .020 | <.0001 | <.0001 | |||||
| Strong alcoholic beveragesa,c n (%) | |||||||||
| Frequently | 15 | (2.2) | 2 | (6.7) | 20 | (11.8) | 13 | (12.2) | |
| Sometimes | 106 | (13.0) | 7 | (15.1) | 34 | (20.1) | 29 | (23.2) | |
| Never | 895 | (84.7) | 41 | (78.2) | 120 | (68.1) | 64 | (64.6) | <.0001 |
| P vs controls | – | .23 | <.0001 | <.0001 | |||||
| Smoking (pack-years)a—n (%) | |||||||||
| 0 | 512 | (37.4) | 19 | (22.7) | 26 | (14.8) | 11 | (12.4) | |
| <30 | 219 | (28.2) | 14 | (36.2) | 35 | (19.3) | 17 | (16.6) | |
| ≥30 | 291 | (34.4) | 17 | (41.1) | 113 | (65.9) | 78 | (71.0) | <.0001 |
| P vs controls | – | .066 | <.0001 | <.0001 | |||||
| High-temperature fooda,c—n (%) | |||||||||
| Likes very much | 47 | (4.7) | 8 | (18.4) | 34 | (20.8) | 17 | (20.6) | |
| Likes somewhat | 240 | (21.5) | 14 | (24.5) | 44 | (25.1) | 29 | (24.0) | |
| Neither likes nor dislikes | 590 | (60.0) | 22 | (45.8) | 74 | (41.8) | 42 | (36.8) | |
| Dislikes somewhat | 110 | (9.8) | 5 | (9.3) | 18 | (9.9) | 15 | (15.9) | |
| Dislikes very much | 25 | (3.9) | 1 | (2.0) | 4 | (2.3) | 3 | (2.7) | <.0001 |
| P vs controls | – | .008 | <.0001 | .0008 | |||||
| Green and yellow vegetablesa,c—n (%) | |||||||||
| Seldom | 7 | (0.5) | 0 | (0.0) | 6 | (3.8) | 2 | (2.1) | |
| 1–2 d/mo | 24 | (1.8) | 2 | (3.8) | 10 | (5.7) | 7 | (5.5) | |
| 1–2 d/wk | 187 | (16.9) | 9 | (20.5) | 29 | (16.5) | 37 | (35.3) | |
| 3–4 d/wk | 346 | (36.2) | 12 | (27.7) | 49 | (29.0) | 31 | (28.4) | |
| Almost every day | 455 | (44.7) | 27 | (48.0) | 80 | (45.1) | 29 | (28.7) | <.0001 |
| P vs controls | – | .76 | .030 | <.0001 | |||||
| Fruita,c—n (%) | |||||||||
| Seldom | 38 | (2.2) | 5 | (11.1) | 16 | (9.6) | 16 | (12.8) | |
| 1–2 d/mo | 63 | (5.9) | 2 | (5.2) | 25 | (15.2) | 14 | (12.6) | |
| 1–2 d/wk | 230 | (20.7) | 15 | (33.4) | 39 | (22.9) | 34 | (33.1) | |
| 3–4 d/wk | 227 | (22.8) | 13 | (26.2) | 35 | (20.2) | 18 | (17.0) | |
| Almost every day | 450 | (48.5) | 15 | (24.1) | 59 | (32.0) | 24 | (24.5) | <.0001 |
| P vs controls | – | .0002 | <.0001 | <.0001 | |||||
| Alcohol flushinga,c—n (%) | |||||||||
| Never flushing | 541 | (45.2) | 20 | (35.2) | 56 | (31.5) | 44 | (40.6) | |
| Flushing | 475 | (54.8) | 30 | (64.8) | 118 | (68.5) | 62 | (59.4) | .0005 |
| P vs controls | – | .23 | <.0001 | <.0001 | |||||
SD, standard deviation.
Percentage values were adjusted for sex and age by a direct method (using all cancer cases as the standard population) and evaluated statistically using the Cochran–Mantel–Haenszel test. Mean values were adjusted for sex and age and statistically tested using analysis of covariance (ANCOVA).
Never/rare, <1 unit/wk; light, 1–8.9 units/wk; moderate, 9–17.9 units/wk; heavy, ≥18 units/wk (1 unit = 22 grams of ethanol).
The number of controls does not add up to 1022 because of missing values.
Risk Factors for Field Cancerization
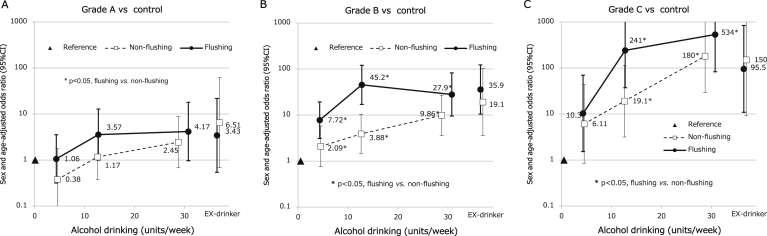
Figure 4 shows the associations between alcohol consumption, flushing reactions, and OR for each LVL grade adjusted for sex and age. Increases in the amount of alcohol drinking were associated with increases in the risk of development of the LVL in all LVL grades, especially in the flushing group. However, in the grade A LVL, no significant differences (ns) were observed between the flushing and no-flushing groups: light, OR = 1.06 (95% CI, 0.32–3.57) vs 0.38 (95% CI, 0.08–1.78; ns for flushing vs no-flushing); moderate, OR = 3.57 (95% CI, 1.00–12.8) vs 1.17 (95% CI, 0.38–3.61; ns); and heavy, OR = 4.17 (95% CI, 0.97–18.0) vs 2.45 (95% CI, 0.68–8.85; ns). In contrast, significant differences were observed in the grade B LVL: light, OR = 7.72 (95% CI, 3.10–19.3) vs 2.09 (95% CI, 0.76–5.76; P < .05); moderate, OR = 45.2 (95% CI, 17.0–120) vs 3.88 (95% CI, 1.45–0.4; P < .05); and heavy, OR = 27.9 (95% CI, 9.49–82.1) vs 9.86 (95% CI, 3.60–27.0; P < .05) and in the grade C LVL: light OR = 10.3 (95% CI, 1.53–69.7) vs 6.11 (95% CI, 0.85–43.9; ns), moderate OR = 241 (95% CI, 37.3–999; P < .05) vs 19.1 (95% CI, 3.16–115; P < .05); and heavy OR = 534 (95% CI, 82.9–<999) vs 180 (95% CI, 29.3–999<; P < .05). In the ex-drinking group, although there was no significant difference in the LVL grade between the flushing and no-flushing groups, the ORs tended to increase along with an increased LVL grade.
Figure 4.
Alcohol consumption, flushing reaction, and odds ratios (ORs) for each LVL group adjusted for sex and age. Panels A, B, and C show the relationship between alcohol drinking and the LVL grade as per flushing reaction. The white boxes and black circles show the sex- and age-adjusted ORs of exhibiting the LVL for light (1–8.9 units/wk), moderate (9–17.9 units/wk), or heavy alcohol drinkers (≥18 units/wk) and for ex-drinkers in the patients with no-flushing and flushing reactions, respectively; the vertical lines show the 95% CIs; the black triangle shows the reference category who are nondrinkers regardless of flushing reaction. The asterisk (∗) indicates a statistically significant difference in OR between the patients with no-flushing reaction and those with flushing reactions in the same category of alcohol consumption.
Cumulative Second Primary ESCC and HNSCC
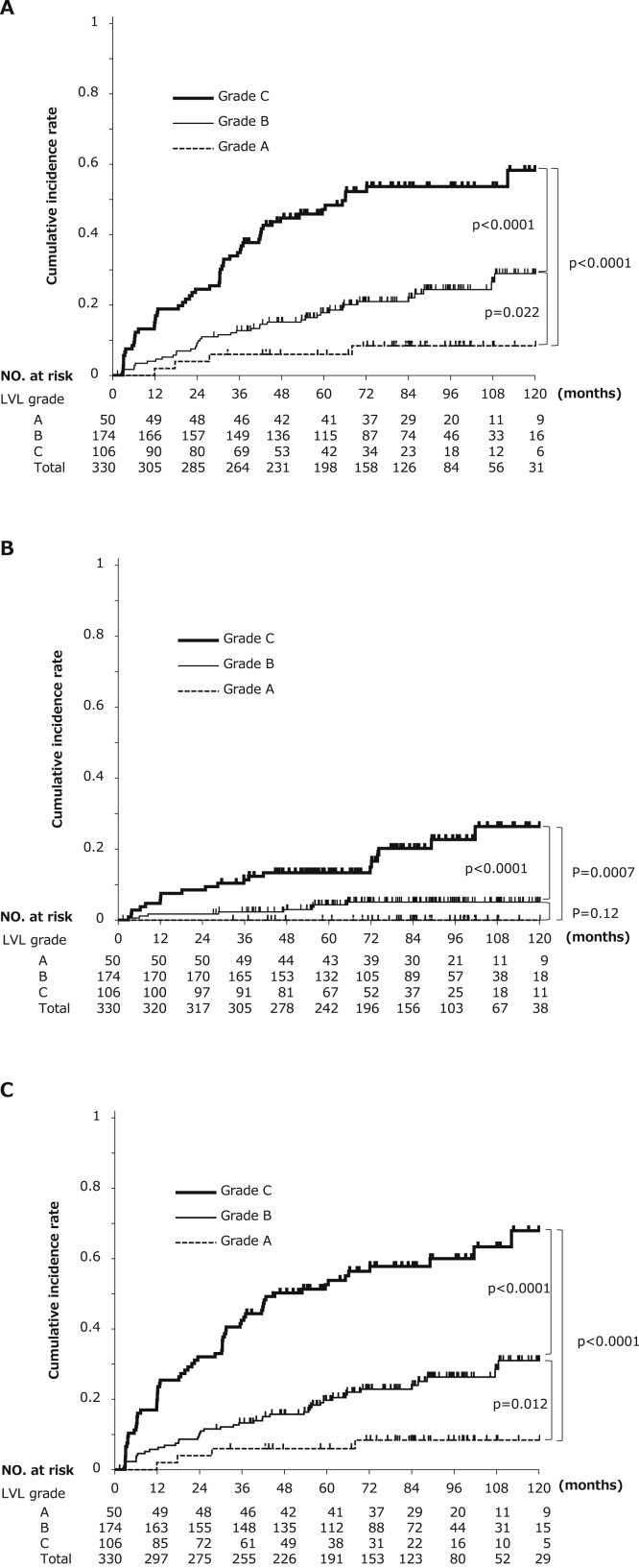
The median follow-up period was 80.7 months (range 1.3–142.3). LVL grades (A–C) were significantly associated with progressive increases in the 5-year cumulative incidence of second primary ESCC and HNSCC (Figure 5A and B): esophagus 6.0%, 17.8%, and 47.1%, respectively; P = .022 for A vs B and P < .0001 for A vs C; head and neck, 0.0%, 4.3%, and 13.3%, respectively, P = .12 for A vs B and P = .0007 for A vs C; esophagus or head and neck, 6.0%, 19.8%, and 52.6%, respectively, P = .012 for A vs B and P < .0001 for A vs C. The combined cumulative incidence of second primary ESCC and HNSCC is shown in Figure 5C.
Figure 5.
Cumulative incidences of second primary ESCC and HNSCC arising from field cancerization as per the grade of the LVL. Panels A and B show cumulative incidences of second primary ESCC and HNSCC as per the grade of the LVL (A–C), respectively. Panel C shows the combined cumulative incidences of second primary ESCC and HNSCC as per the grade of the LVL (A–C). The P values are for differences in the cumulative incidence between LVL grades A vs B, B vs C, and A vs C by the log–rank test.
Effect of Cessation of Drinking and Smoking
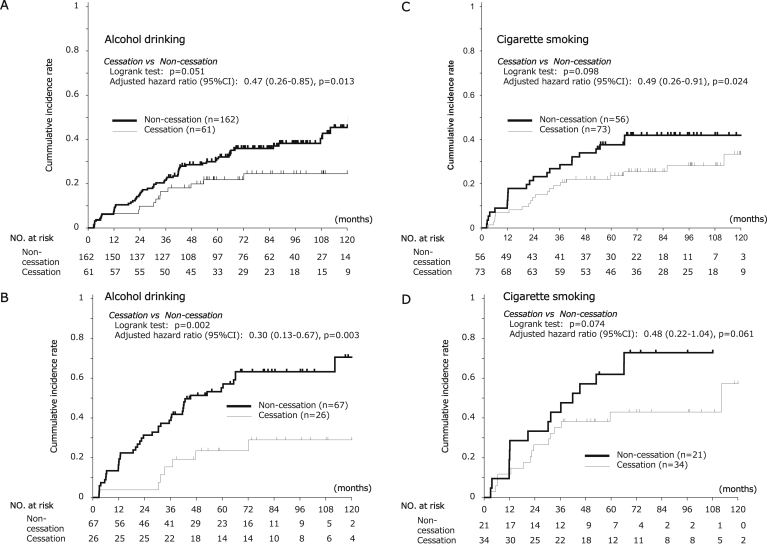
One hundred seven patients were nondrinkers or ex-drinkers at registration. Two hundred one patients were nonsmokers or ex-smokers at registration. These patients were not suitable for the analysis of effect of cessation of drinking and smoking during the surveillance. Then, we evaluated the effect of cessation of drinking and smoking in remaining 223 patients and 129 patients, respectively. As shown in Figure 5, 61 and 73 patients completed cessation of alcohol and cigarette use during the surveillance period, whereas 162 and 56 did not, respectively. Alcohol and smoking cessation decreased the risk of a second primary ESCC (adjusted HRs, 0.47 and 0.49, respectively, P = .013 for alcohol and P = .024 for smoking, Figure 6A and C). The risk reduction for a second primary ESCC by cessation of drinking and smoking was particularly large in the grade C LVL (cessation drinking: adjusted HR, 0.30; 95% CI, 0.13–0.67; P = .003; cessation of smoking: adjusted HR, 0.48; 95% CI, 0.22–1.04; P = .061, Figure 6B and D).
Figure 6.
Cumulative incidence of second primary ESCC and HNSCC arising from field cancerization associated with cessation of drinking or smoking. Panel A shows the cumulative incidences of second primary ESCC in all patients classed as alcohol drinkers at registration. Panel B shows the cumulative incidences of second primary ESCC in the patients with a grade C LVL. Panel C shows the cumulative incidences of second primary ESCC in all patients classed as smokers at registration. Panel D shows the cumulative incidences of second primary ESCC in the patients with a grade C LVL. The HRs were adjusted for sex, age, LVL grade, numbers of cigarettes a day, pack-years, amount of alcohol drinking at baseline, and alcohol flushing reaction. The P values are for the differences in cumulative incidence between the drinking and drinking-cessation groups and between the smoking and smoking-cessation groups.
Discussion
The field cancerization phenomenon was first proposed in 1953 to explain the multiple development of SCC in the upper aerodigestive tract and was associated with alcohol and cigarette use.7 However, how they affect the expansion of squamous dysplastic epithelium and how cessation affects developing second primary ESCC and HNSCC after curative treatment are not clearly elucidated. In other words, although alcohol and cigarette use are well known as carcinogens, effective strategies for preventing these cancers have not been established. In particular, recent advances in endoscopic technology enable the detection of these cancers at early stages, enabling prolonged survival, and minimally invasive treatment can help preserve the organs. For such cancer survivors, effective prevention and surveillance for second primary cancers are clinically essential.
Acetaldehyde is an obvious carcinogen for ESCC and HNSCC; however, how acetaldehyde is involved in this carcinogenesis has not been clear. In our study, we could show that acetaldehyde-derived DNA adducts were accumulated by drinking alcohol, and DNA adducts were decreased by abstinence from alcohol in the mice model. The fact in the mice model that the amount of DNA adducts varies greatly depending on the ALDH2 genotype might clearly indicate that acetaldehyde is involved in some kind of cancer initiation. Furthermore, in humans, the amount of alcohol consumption correlated positively with the amount of acetaldehyde-derived DNA adducts. These data indicate that alcohol consumption can cause esophageal carcinogenesis. However, the esophageal DNA adduct level in healthy subjects was not affected by the ALDH2 genotype. Although the reason of this result is unknown, one possibility is that it is difficult to match the conditions for drinking in each healthy subject. Actually, we already reported that the half-life of N2-ethylidene-dG was 35 hours.23 This means that most of the DNA adducts could disappear before the subjects visit to the hospital unless we ask them to continue drinking. Another possibility was the differences in alcohol consumption volume between mice and humans. In the mice model, ALDH2-WT mice drank 0.32 g ethanol per day, and inactive ALDH2 mice drank 0.21 g per day. When converted to a human weighing 60 kg (multiplied by 2000), this means that humans will drink about 8–12 liters of 5% beer per day. This is an unrealistic amount for humans. In addition, as the number of heavy drinkers was small in this analysis, it is possible that there was not a large difference. In patients with esophageal cancer, while we could not detect DNA adducts in the mucosa of the grade A LVL, we also could detect DNA adducts in some mucosa of the grade C LVL. In a clinical perspective, most patients with ESCC refrain from drinking before their visit to the hospital. This situation might be similar to the healthy subject because the patients were strongly instructed to stop drinking and quit smoking from the time of diagnosis. On the other hand, we detected a high DNA adduct level in the mucosa of the grade C LVL of patients who were unknown for abstinence from alcohol and smoking. The important evidence was that acetaldehyde-derived DNA adducts could be detected in the human esophagus. We believe that this exploratory result needs to be prospectively examined in a large number of cases.
This study is unique in that it focuses on the development of dysplastic squamous epithelium, whereas most studies have focused on the development of cancer itself, and we believe that this study will provide important data for the field carcinogenesis. From a clinical perspective, herein, we showed that among patients with newly diagnosed superficial ESCC, LVL grades were positively associated with the intensity of alcohol drinking, flushing reactions, smoking, and consuming high-temperature food and negatively associated with the consumption of green-yellow vegetables and fruit. In particular, the increased risk of progression of LVL grade was associated with the amount of alcohol consumption and with flushing reactions. The greatest risk was observed in the patients with flushing reactions who consumed an average of 30 units per week in the grade C LVL. The OR was 534 (95% CI, 82.9–<999) compared with healthy subjects. This suggests that alcohol is a strong carcinogen especially for patients with a flushing reaction. Because flushing reaction is caused by accumulation of acetaldehyde due to ALDH2 deficient, our result also means that acetaldehyde is a strong carcinogen in field cancerization.
In the general population, it takes more than 20 years to reduce the risk of ESCC and HNSCC after ceasing alcohol consumption.24,25 However, the effect of stopping alcohol and cigarette use on field cancerization has not been clear. Most of the patients in our study were more than 60 years old. Therefore, asking them to abstain for more than 20 years is unrealistic because they will be not able to experience the benefit during their expected life duration. If short-term beneficial effects could be demonstrated, these patients might understand and try to reduce the risk of second primary cancers. Our present data showed that at least 5-year cessation of drinking significantly reduced the risk of developing second primary ESCC, especially among the patients with a grade C LVL.
Smoking cessation was reported to decrease the risk of developing ESCC in a time-dependent manner, particularly in Western populations.26 In that study, compared with current smokers, a strong risk reduction was evident after ≥5 years (relative risk, 0.59; 95% CI, 0.47–0.75) and became stronger after ≥10 years (relative risk, 0.34; 95% CI, 0.25–0.47) after smoking cessation. However, the evidence has been weak in Asian populations. Here, we found that at least 5-year cessation of smoking significantly reduced the risk of developing a second primary ESCC. Thus, cessation of smoking will reduce the risk of field cancerization even in the short term and in Asian populations.
The mechanism of carcinogenesis by alcohol and cigarette use has not been clearly elucidated. We have reported that driver-mutated clones of cells in the physiologically normal esophageal epithelium emerged multifocally from early childhood and increased their number and size with aging and ultimately replaced almost the entire esophageal epithelium in extremely old people.9 Moreover, such driver-mutated clones were increased significantly in numbers with heavy smoking and drinking. These phenomena could be explained by the field effect and were identified clinically as multiple LVLs. Conversely, we cannot escape age-related remodeling of esophageal epithelium by mutated cancer drivers, but we can avoid or minimize the acceleration caused by drinking and smoking. Our data support this strategy credible and can help reduce the risk of field cancerization. In fact, we found that cessation of alcohol and cigarette use could improve LVL status.27 This means that cessation of alcohol and smoking inhibits the expansion of squamous dysplastic epithelium and finally reduces the development of second primary cancers.
Our study had several limitations. First, we did not randomize the patients to cessation and noncessation groups. However, alcohol and smoking are well-known carcinogens in various cancers. Therefore, a noncessation group is difficult to set up from the viewpoint of ethics. Second, the cohort part of this study included only patients with cancer because our research target was second primary cancers developing after curative treatments. We believe that our data will be useful to establish a prevention and surveillance strategy for cancer survivors because the overall prognosis of esophageal cancer and head and neck cancer is still poor (<20% 5-year survival).
In conclusion, this study showed significant positive associations of alcohol consumption, cigarette smoking, and high-temperature food with field cancerization, especially for patients with a flushing reaction. In particular, the increased risk of expansion of the LVL was associated with the amount of alcohol consumption and with flushing reactions. In contrast, consumption of green-yellow vegetables and fruit reduces the risk of field cancerization. Furthermore, cessation of both drinking and smoking could reduce the incidences of second primary ESCC after curative treatment. Our results emphasize the importance of education regarding the cessation of drinking and smoking for cancer survivors.
Acknowledgments
The authors thank all the investigators of the Japan Esophageal Cohort study at 16 participating hospitals: Kazuhiro Kaneko, MD, PhD, Shuko Morita, MD, Makiko Funakoshi MD, Takahiro Horimatsu, MD, PhD, Mari Takahashi, MS, Kazuhiro Kaneko, MD, PhD, Haruhisa Suzuki, MD, PhD, Satoshi Abiko, MD, PhD, Kenichi Takemura, MD, PhD, Hiroyoshi Nakanishi, MD, PhD, Masahiro Saito, MD, PhD, Nobuyuki Ara, MD, PhD, Naomi Kakushima, MD, PhD, Masaki Tanaka, MD, Keisuke Hori, MD, PhD, and Takashi Tsuda MD, PhD. They also thank the data management team at the Medical Research Support (Osaka, Japan): Tomoko Aoyama and Minae Nishiguchi.
Authors' Contributions
Manabu Muto, Chikatoshi Katada, Tomonori Yano, and Akira Yokoyama contributed to study concept and design. Manabu Muto, Chikatoshi Katada, Tetsuji Yokoyama, Ichiro Oda, Yasumasa Ezoe, Satoshi Tanabe, Yuichi Shimizu, Hisashi Doyama, Tomoyuki Koike, Kohei Takizawa, Motohiro Hirao, Hiroyuki Okada, Takashi Ogata, Atsushi Katagiri, Takenori Yamanouchi, Yasumasa Matsuo, Hirofumi Kawakubo, Tai Omori, Nozomu Kobayashi, and Akira Yokoyama contributed to acquisition of clinical data. Kiichiro Baba, Akira Yokoyama, and Shinya Ohashi contributed to acquisition of in vitro and in vivo data. Manabu Muto, Tai Omori, and Kazuhiro Kaneko contributed to central review for grading of Lugol-voiding lesions. Tadakazu Shimoda and Atsushi Ochiai contributed to pathological advising. Manabu Muto, Chikatoshi Katada, Hideki Ishikawa, and Tetsuji Yokoyama contributed to statistical analysis. Manabu Muto, Chikatoshi Katada, and Tetsuji Yokoyama interpreted the data. All authors reviewed and gave final approval of the literature. Manabu Muto drafted the manuscript.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest relevant to this study.
Funding: This work was supported by the grant from National Cancer Center Research and Development Fund 36 by the Ministry of Health, Labour and Welfare of Japan.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: Data will be made available on reasonable request via the corresponding author.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2021.10.005.
Contributor Information
Manabu Muto, Email: mmuto@kuhp.kyoto-u.ac.jp.
Japan Esophageal Cohort Study Group:
Kazuhiro Kaneko, Shuko Morita, Makiko Funakoshi, Takahiro Horimatsu, Mari Takahashi, Kazuhiro Kaneko, Haruhisa Suzuki, Satoshi Abiko, Kenichi Takemura, Hiroyoshi Nakanishi, Masahiro Saito, Nobuyuki Ara, Naomi Kakushima, Masaki Tanaka, Keisuke Hori, and Takashi Tsuda
Supplementary Materials
References
- 1.International Agency for Research on Cancer . International Agency for Research on Cancer; Lyon, France: 1988. Alcohol drinking. [Google Scholar]
- 2.International Agency for Research on Cancer . International Agency for Research on Cancer; Lyon, France: 1986. Tobacco smoking. [Google Scholar]
- 3.Holmes R.S., Vaughan T.L. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Enzinger P.C., Mayer R.J. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 5.Hashibe M., Brennan P., Chuang S.C., et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor B., Rehm J. When risk factors combine: the interaction between alcohol and smoking for aerodigestive cancer, coronary heart disease, and traffic and fire injury. Addict Behav. 2006;31:1522–1535. doi: 10.1016/j.addbeh.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Slaughter D.P., Southwick H.W., Smejkal W. “Field cancerization” in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Muto M., Takahashi M., Ohtsu A., et al. Risk of multiple squamous cell carcinomas both in the esophagus and the head and neck region. Carcinogenesis. 2005;26:1008–1012. doi: 10.1093/carcin/bgi035. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama A., Kakiuchi N., Yoshizawa T., et al. Age-related remodeling of oesophageal epithelia by mutated cancer drivers. Nature. 2019;565:312–317. doi: 10.1038/s41586-018-0811-x. [DOI] [PubMed] [Google Scholar]
- 10.Muto M., Hitomi Y., Ohtsu A., et al. Association of aldehyde dehydrogenase 2 gene polymorphism with multiple oesophageal dysplasia in head and neck cancer patients. Gut. 2000;47:256–261. doi: 10.1136/gut.47.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoyama T., Yokoyama A., Kato H., et al. Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes, and risk for esophageal squamous cell carcinoma in Japanese men. Cancer Epidemiol Biomarkers Prev. 2003;12:1227–1233. [PubMed] [Google Scholar]
- 12.Yokoyama A., Kato H., Yokoyama T., et al. Esophageal squamous cell carcinoma and aldehyde dehydrogenase-2 genotypes in Japanese females. Alcohol Clin Exp Res. 2006;30:491–500. doi: 10.1111/j.1530-0277.2006.00053.x. [DOI] [PubMed] [Google Scholar]
- 13.Katada C., Yokoyama T., Yano T., et al. Alcohol consumption and multiple dysplastic lesions increase risk of squamous cell carcinoma in the esophagus, head, and neck. Gastroenterology. 2016;151:560–569. doi: 10.1053/j.gastro.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Secretan B., Straif K., Baan R., et al. A review of human carcinogens-part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 15.Reinskje T., Antoon O., Jan G.C., et al. Role of acetaldehyde in tobacco smoke addiction. Eur Neuropsychopharmacol. 2007;17:627–636. doi: 10.1016/j.euroneuro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Salaspuro V., Hietala J., Kaihovaara P., et al. Removal of acetaldehyde from saliva by a slow release buccal tablet of L-cysteine. Int J Cancer. 2002;97:361–364. doi: 10.1002/ijc.1620. [DOI] [PubMed] [Google Scholar]
- 17.Salaspuro V.J., Hietala J.M., Marvola M.L., et al. Eliminating carcinogenic acetaldehyde by cysteine from saliva during smoking. Cancer Epidemiol Biomarkers Prev. 2006;15:146–149. doi: 10.1158/1055-9965.EPI-05-0248. [DOI] [PubMed] [Google Scholar]
- 18.Kitagawa K., Kawamoto T., Kunugita N., et al. Aldehyde dehydrogenase (ALDH) 2 associates with oxidation ofmethoxyacetaldehyde; in vitro analysis with liver subcellular fractionderived from human and Aldh2 gene targeting mouse. FEBS Lett. 2000;476:306–311. doi: 10.1016/s0014-5793(00)01710-5. [DOI] [PubMed] [Google Scholar]
- 19.Amanuma Y., Ohashi S., Itatani Y., et al. Protective role of ALDH2 against acetaldehyde-derived DNA damage in oesophageal squamous epithelium. Sci Rep. 2016;5:14142. doi: 10.1038/srep14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada H., Nakagawa H., Oyama K., et al. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a Inactivation. Mol Cancer Res. 2003;1:729–738. [PubMed] [Google Scholar]
- 21.Blokzijl F., Janssen R., Van Boxtel R., et al. Mutational patterns: an integrative R package for studying patterns in base substitution catalogues. Genome Med. 2018;10:33. doi: 10.1186/s13073-018-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks P.J., Enoch M.A., Goldman D., et al. The Alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e1000050. doi: 10.1371/journal.pmed.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hori K., Miyamoto S., Yukawa Y., et al. Stability of acetaldehyde-derived DNA adduct in vitro. Biochem Biophys Res Commun. 2012;423:624–646. doi: 10.1016/j.bbrc.2012.05.158. [DOI] [PubMed] [Google Scholar]
- 24.LoConte N.K., Brewster A.M., Kaur J.S., et al. Alcohol and cancer: a statement of the American Society of Clinical Oncology. J Clin Oncol. 2017;36:83–93. doi: 10.1200/JCO.2017.76.1155. [DOI] [PubMed] [Google Scholar]
- 25.Kiadaliri A.A., Jarl J., Gavriilidis G., et al. Alcohol drinking cessation and the risk of laryngeal and pharyngeal cancers: a systematic review and meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q.L., Xie S.H., Li W.T., et al. Smoking cessation and risk of esophageal cancer by histological type: systematic review and meta-analysis. J Natl Cancer Inst. 2017;109:djx115. doi: 10.1093/jnci/djx115. [DOI] [PubMed] [Google Scholar]
- 27.Hori K., Okada H., Konishi K., et al. Continuously cessation or reduction of drinking habit improves the Lugol-voiding legions in patients of esophageal squamous cell carcinoma after endoscopic resection. Gastrointest Endosc. 2015;81(Suppl):AB519. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.