Abstract
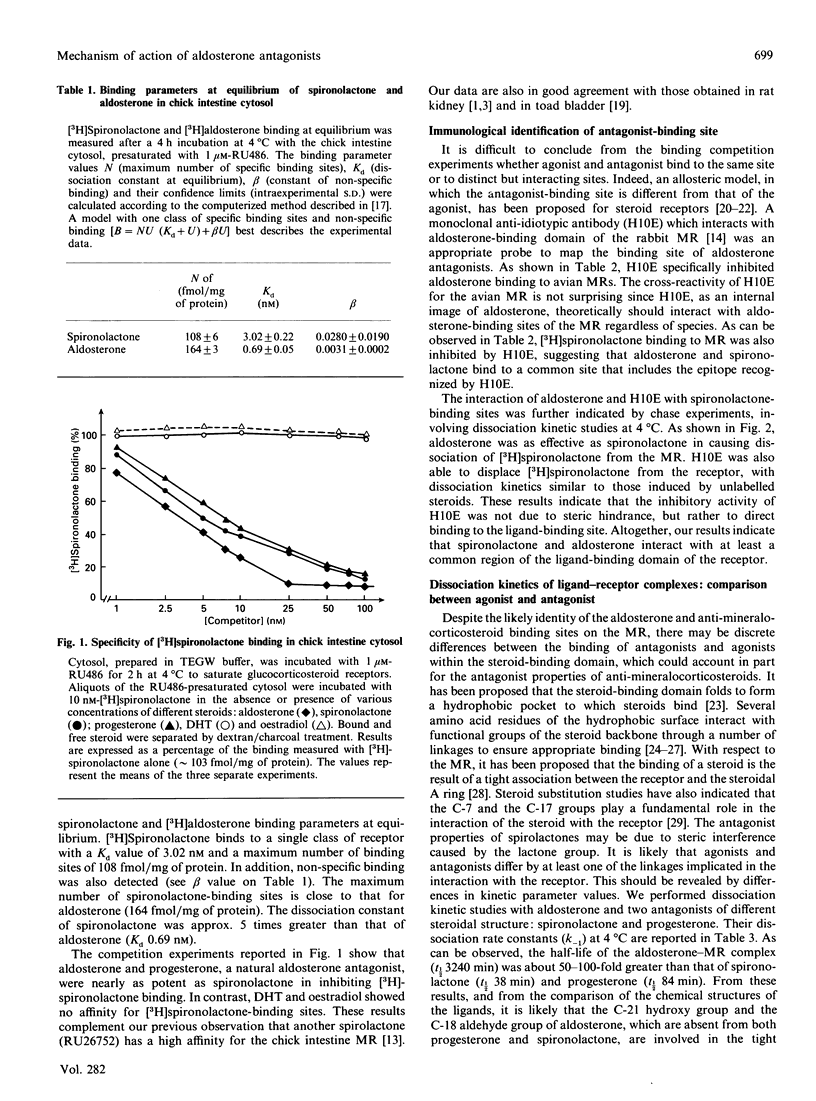
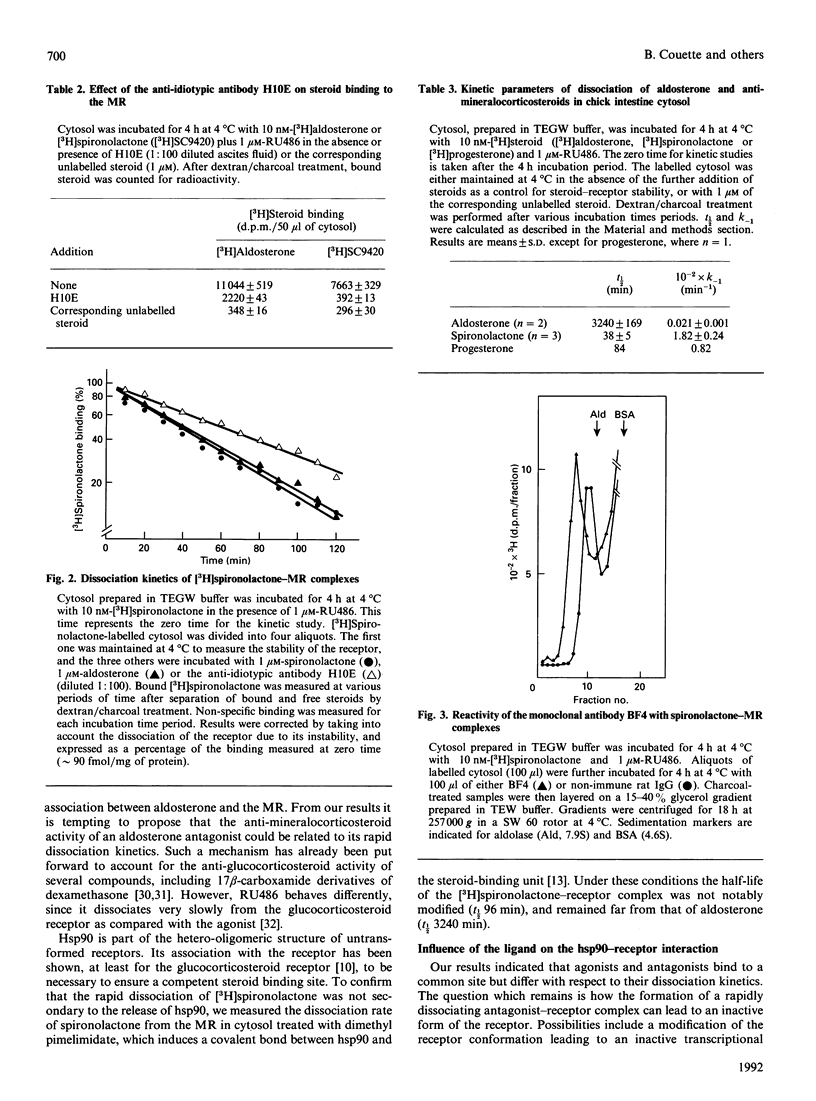
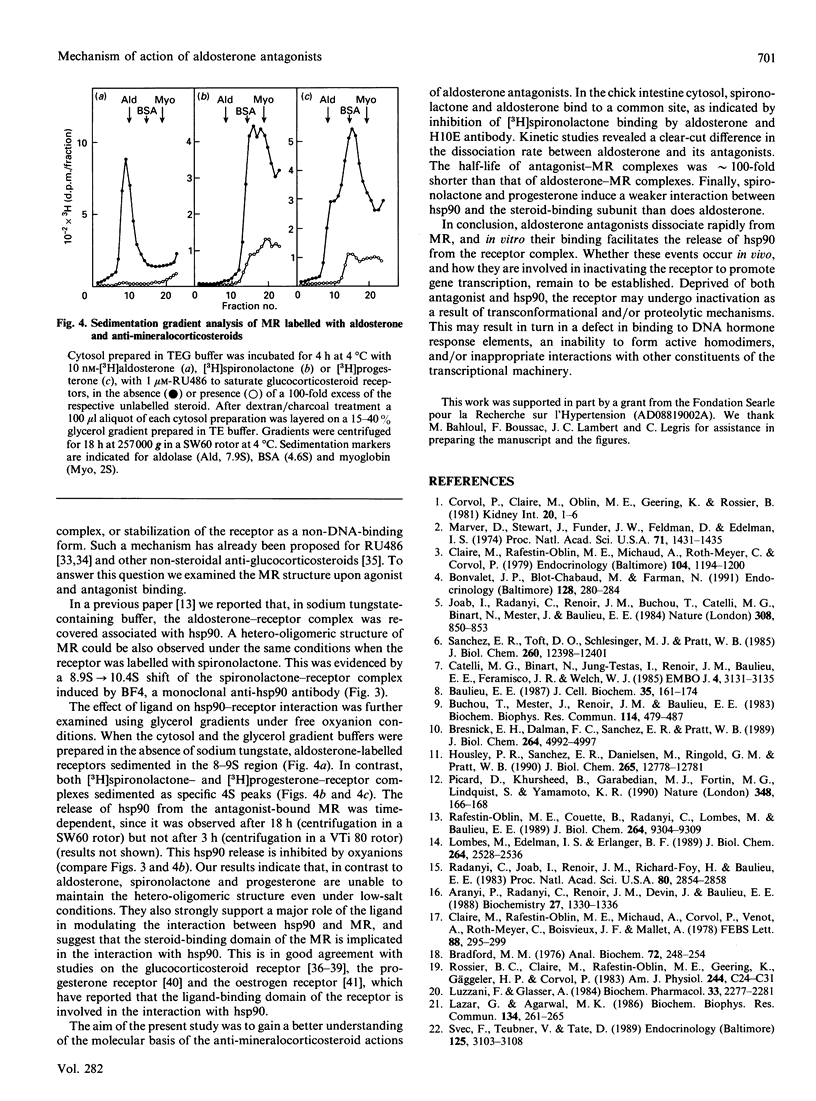
To elucidate the mechanism of action of aldosterone antagonists, we studied the interaction of spironolactone with the chick mineralocorticosteroid receptor (MR). Intestinal cytosol contains specific spironolactone-binding sites (Kd approximately 3 nM; max. no. of binding sites approximately 100 fmol/mg of protein) that have been identified as MRs by competition experiments with steroid ligands and with the monoclonal anti-idiotypic antibody H10E that interacts with aldosterone-binding domain of the MR. Binding studies indicate that aldosterone and spironolactone bind to the MR through a common site that encompasses the epitope recognized by H10E. At 4 degrees C, spironolactone dissociates much more rapidly from the cytosol 8-9 S form of MR (t1/2 38 min) than does aldosterone (t1/2 3240 min). A high dissociation rate was also observed for progesterone, a natural aldosterone antagonist (t1/2 84 min). The covalent linkage of the 90 kDa heat shock protein (hsp90) to the ligand-binding subunit of MR with dimethyl pimelimidate did not notably modify the rate of dissociation of spironolactone from the receptor (t1/2 96 min), excluding the possibility that the rapid dissociation rate of the antagonist was related to hsp90 release. The effects of aldosterone and the two anti-mineralocorticosteroids on the 8-9 S heterooligomeric structure of the MR differed strikingly. Using low-salt density-gradient centrifugation analysis, aldosterone-labelled receptors were recovered as 8-9S complexes, whereas 4 S entities were detected after spironolactone and progesterone binding. This indicated that, under the experimental conditions used, aldosterone antagonists facilitate hsp90 release and thus do not stabilize the non-DNA-binding 8-9S form of MR. We propose that the combination of rapid dissociation of the ligand and a weakened hsp90-receptor interaction is involved in the anti-mineralococorticosteroid activity of aldosterone antagonists.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arànyi P., Radanyi C., Renoir M., Devin J., Baulieu E. E. Covalent stabilization of the nontransformed chick oviduct cytosol progesterone receptor by chemical cross-linking. Biochemistry. 1988 Feb 23;27(4):1330–1336. doi: 10.1021/bi00404a036. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E. Steroid hormone antagonists at the receptor level: a role for the heat-shock protein MW 90,000 (hsp 90). J Cell Biochem. 1987 Oct;35(2):161–174. doi: 10.1002/jcb.240350209. [DOI] [PubMed] [Google Scholar]
- Bonvalet J. P., Blot-Chabaud M., Farman N. Autoradiographic evidence of nuclear binding of spironolactone in rabbit cortical collecting tubule. Endocrinology. 1991 Jan;128(1):280–284. doi: 10.1210/endo-128-1-280. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bresnick E. H., Dalman F. C., Sanchez E. R., Pratt W. B. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J Biol Chem. 1989 Mar 25;264(9):4992–4997. [PubMed] [Google Scholar]
- Buchou T., Mester J., Renoir J. M., Baulieu E. E. Effects of urea and molybdate on the chick oviduct progesterone receptor. Biochem Biophys Res Commun. 1983 Jul 29;114(2):479–487. doi: 10.1016/0006-291x(83)90805-7. [DOI] [PubMed] [Google Scholar]
- Cadepond F., Schweizer-Groyer G., Segard-Maurel I., Jibard N., Hollenberg S. M., Giguère V., Evans R. M., Baulieu E. E. Heat shock protein 90 as a critical factor in maintaining glucocorticosteroid receptor in a nonfunctional state. J Biol Chem. 1991 Mar 25;266(9):5834–5841. [PubMed] [Google Scholar]
- Carlstedt-Duke J., Strömstedt P. E., Persson B., Cederlund E., Gustafsson J. A., Jörnvall H. Identification of hormone-interacting amino acid residues within the steroid-binding domain of the glucocorticoid receptor in relation to other steroid hormone receptors. J Biol Chem. 1988 May 15;263(14):6842–6846. [PubMed] [Google Scholar]
- Carson-Jurica M. A., Lee A. T., Dobson A. W., Conneely O. M., Schrader W. T., O'Malley B. W. Interaction of the chicken progesterone receptor with heat shock protein (HSP) 90. J Steroid Biochem. 1989;34(1-6):1–9. doi: 10.1016/0022-4731(89)90060-5. [DOI] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Jung-Testas I., Renoir J. M., Baulieu E. E., Feramisco J. R., Welch W. J. The common 90-kd protein component of non-transformed '8S' steroid receptors is a heat-shock protein. EMBO J. 1985 Dec 1;4(12):3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambraud B., Berry M., Redeuilh G., Chambon P., Baulieu E. E. Several regions of human estrogen receptor are involved in the formation of receptor-heat shock protein 90 complexes. J Biol Chem. 1990 Nov 25;265(33):20686–20691. [PubMed] [Google Scholar]
- Claire M., Rafestin-Oblin M. E., Michaud A., Corvol P., Venot A., Roth-Meyer C., Boisvieux J. F., Mallet A. Statistical test of models and computerised parameter estimation for aldosterone binding in rat kidney. FEBS Lett. 1978 Apr 15;88(2):295–299. doi: 10.1016/0014-5793(78)80197-5. [DOI] [PubMed] [Google Scholar]
- Claire M., Rafestin-Oblin M. E., Michaud A., Roth-Meyer C., Corvol P. Mechanism of action of a new antialdosterone compound, prorenone. Endocrinology. 1979 Apr;104(4):1194–1200. doi: 10.1210/endo-104-4-1194. [DOI] [PubMed] [Google Scholar]
- Corvol P., Claire M., Oblin M. E., Geering K., Rossier B. Mechanism of the antimineralocorticoid effects of spirolactones. Kidney Int. 1981 Jul;20(1):1–6. doi: 10.1038/ki.1981.97. [DOI] [PubMed] [Google Scholar]
- Denis M., Gustafsson J. A., Wikström A. C. Interaction of the Mr = 90,000 heat shock protein with the steroid-binding domain of the glucocorticoid receptor. J Biol Chem. 1988 Dec 5;263(34):18520–18523. [PubMed] [Google Scholar]
- Groyer A., Schweizer-Groyer G., Cadepond F., Mariller M., Baulieu E. E. Antiglucocorticosteroid effects suggest why steroid hormone is required for receptors to bind DNA in vivo but not in vitro. Nature. 1987 Aug 13;328(6131):624–626. doi: 10.1038/328624a0. [DOI] [PubMed] [Google Scholar]
- Housley P. R., Sanchez E. R., Danielsen M., Ringold G. M., Pratt W. B. Evidence that the conserved region in the steroid binding domain of the glucocorticoid receptor is required for both optimal binding of hsp90 and protection from proteolytic cleavage. A two-site model for hsp90 binding to the steroid binding domain. J Biol Chem. 1990 Aug 5;265(22):12778–12781. [PubMed] [Google Scholar]
- Howard K. J., Holley S. J., Yamamoto K. R., Distelhorst C. W. Mapping the HSP90 binding region of the glucocorticoid receptor. J Biol Chem. 1990 Jul 15;265(20):11928–11935. [PubMed] [Google Scholar]
- Joab I., Radanyi C., Renoir M., Buchou T., Catelli M. G., Binart N., Mester J., Baulieu E. E. Common non-hormone binding component in non-transformed chick oviduct receptors of four steroid hormones. 1984 Apr 26-May 2Nature. 308(5962):850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- Lazar G., Agarwal M. K. Evidence for an antagonist specific receptor that does not bind mineralocorticoid agonists. Biochem Biophys Res Commun. 1986 Jan 14;134(1):261–265. doi: 10.1016/0006-291x(86)90556-5. [DOI] [PubMed] [Google Scholar]
- Lefebvre P., Danze P. M., Sablonniere B., Richard C., Formstecher P., Dautrevaux M. Association of the glucocorticoid receptor binding subunit with the 90K nonsteroid-binding component is stabilized by both steroidal and nonsteroidal antiglucocorticoids in intact cells. Biochemistry. 1988 Dec 27;27(26):9186–9194. doi: 10.1021/bi00426a017. [DOI] [PubMed] [Google Scholar]
- Lefebvre P., Formstecher P., Rousseau G. G., Lustenberger P., Dautrevaux M. Improvement in glucocorticoid receptor binding affinity concomitant to shift from antagonist to agonist activity in a series of 17 beta-carboxamide derivatives of dexamethasone. J Steroid Biochem. 1989 Oct;33(4A):557–563. doi: 10.1016/0022-4731(89)90041-1. [DOI] [PubMed] [Google Scholar]
- Lombes M., Edelman I. S., Erlanger B. F. Internal image properties of a monoclonal auto-anti-idiotypic antibody and its binding to aldosterone receptors. J Biol Chem. 1989 Feb 15;264(5):2528–2536. [PubMed] [Google Scholar]
- Luzzani F., Glässer A. Characterization of spironolactone binding sites distinct from aldosterone receptors in rat kidney homogenates. Biochem Pharmacol. 1984 Jul 15;33(14):2277–2281. doi: 10.1016/0006-2952(84)90667-1. [DOI] [PubMed] [Google Scholar]
- Marver D., Stewart J., Funder J. W., Feldman D., Edelman I. S. Renal aldosterone receptors: studies with (3H)aldosterone and the anti-mineralocorticoid (3H)spirolactone (SC-26304). Proc Natl Acad Sci U S A. 1974 Apr;71(4):1431–1435. doi: 10.1073/pnas.71.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moguilewsky M., Philibert D. RU 38486: potent antiglucocorticoid activity correlated with strong binding to the cytosolic glucocorticoid receptor followed by an impaired activation. J Steroid Biochem. 1984 Jan;20(1):271–276. doi: 10.1016/0022-4731(84)90216-4. [DOI] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Jolly D. J., Pratt D. V., Hollenberg S. M., Giguere V., Cadepond F. M., Schweizer-Groyer G., Catelli M. G., Evans R. M., Baulieu E. E. A region in the steroid binding domain determines formation of the non-DNA-binding, 9 S glucocorticoid receptor complex. J Biol Chem. 1988 Jan 5;263(1):267–273. [PubMed] [Google Scholar]
- Radanyi C., Joab I., Renoir J. M., Richard-Foy H., Baulieu E. E. Monoclonal antibody to chicken oviduct progesterone receptor. Proc Natl Acad Sci U S A. 1983 May;80(10):2854–2858. doi: 10.1073/pnas.80.10.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafestin-Oblin M. E., Couette B., Radanyi C., Lombes M., Baulieu E. E. Mineralocorticosteroid receptor of the chick intestine. Oligomeric structure and transformation. J Biol Chem. 1989 Jun 5;264(16):9304–9309. [PubMed] [Google Scholar]
- Rafestin-Oblin M. E., Lombes M., Lustenberger P., Blanchardie P., Michaud A., Cornu G., Claire M. Affinity of corticosteroids for mineralocorticoid and glucocorticoid receptors of the rabbit kidney: effect of steroid substitution. J Steroid Biochem. 1986 Oct;25(4):527–534. doi: 10.1016/0022-4731(86)90398-5. [DOI] [PubMed] [Google Scholar]
- Rossier B. C., Claire M., Rafestin-Oblin M. E., Geering K., Gäggeler H. P., Corvol P. Binding and antimineralocorticoid activities of spirolactones in toad bladder. Am J Physiol. 1983 Jan;244(1):C24–C31. doi: 10.1152/ajpcell.1983.244.1.C24. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Toft D. O., Schlesinger M. J., Pratt W. B. Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biol Chem. 1985 Oct 15;260(23):12398–12401. [PubMed] [Google Scholar]
- Segnitz B., Gehring U. Mechanism of action of a steroidal antiglucocorticoid in lymphoid cells. J Biol Chem. 1990 Feb 15;265(5):2789–2796. [PubMed] [Google Scholar]
- Simons S. S., Jr, Pumphrey J. G., Rudikoff S., Eisen H. J. Identification of cysteine 656 as the amino acid of hepatoma tissue culture cell glucocorticoid receptors that is covalently labeled by dexamethasone 21-mesylate. J Biol Chem. 1987 Jul 15;262(20):9676–9680. [PubMed] [Google Scholar]
- Smith L. I., Bodwell J. E., Mendel D. B., Ciardelli T., North W. G., Munck A. Identification of cysteine-644 as the covalent site of attachment of dexamethasone 21-mesylate to murine glucocorticoid receptors in WEHI-7 cells. Biochemistry. 1988 May 17;27(10):3747–3753. doi: 10.1021/bi00410a034. [DOI] [PubMed] [Google Scholar]
- Strömstedt P. E., Berkenstam A., Jörnvall H., Gustafsson J. A., Carlstedt-Duke J. Radiosequence analysis of the human progestin receptor charged with [3H]promegestone. A comparison with the glucocorticoid receptor. J Biol Chem. 1990 Aug 5;265(22):12973–12977. [PubMed] [Google Scholar]
- Svec F., Teubner V., Tate D. Location of the second steroid-binding site on the glucocorticoid receptor. Endocrinology. 1989 Dec;125(6):3103–3108. doi: 10.1210/endo-125-6-3103. [DOI] [PubMed] [Google Scholar]


