Abstract
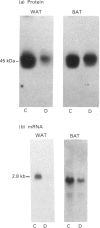
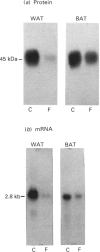
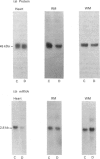
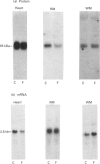
1. GLUT-4 glucose-transporter protein and mRNA levels were assessed in heart, red muscle and white muscle, as well as in brown and white adipose tissue from 7-day streptozotocin-induced diabetic and 48 h-fasted rats. 2. In agreement with previous data, white adipose tissue showed a substantial decrease in GLUT-4 mRNA and protein levels in response to both diabetes and fasting. Similarly, GLUT-4 mRNA and protein markedly decreased in brown adipose tissue in both insulinopenic conditions. 3. Under control conditions, the level of expression of GLUT-4 protein content differed substantially in heart, red and white skeletal muscle. Thus GLUT-4 protein was maximal in heart, and red muscle had a greater GLUT-4 content compared with white muscle. In spite of the large differences in GLUT-4 protein content, GLUT-4 mRNA levels were equivalent in heart and red skeletal muscle. 4. In heart, GLUT-4 mRNA decreased to a greater extent than GLUT-4 protein in response to diabetes and fasting. In contrast, red muscle showed a greater decrease in GLUT-4 protein than in mRNA in response to diabetes or fasting, and in fact no decrease in GLUT-4 mRNA content was detectable in fasting. On the other hand, preparations of white skeletal muscle showed a substantial increase in GLUT-4 mRNA under both insulinopenic conditions, and that was concomitant to either a modest decrease in GLUT-4 protein in diabetes or to no change in fasting. 5. These results indicate that (a) the effects of diabetes and fasting are almost identical and lead to changes in GLUT-4 expression that are tissue-specific, (b) white adipose tissue, brown adipose tissue and heart respond similarly to insulin deficiency by decreasing GLUT-4 mRNA to a larger extent than GLUT-4 protein, and (c) red and white skeletal muscle respond to insulinopenic conditions in a heterogeneous manner which is characterized by enhanced GLUT-4 mRNA/protein ratios.
Full text
PDF
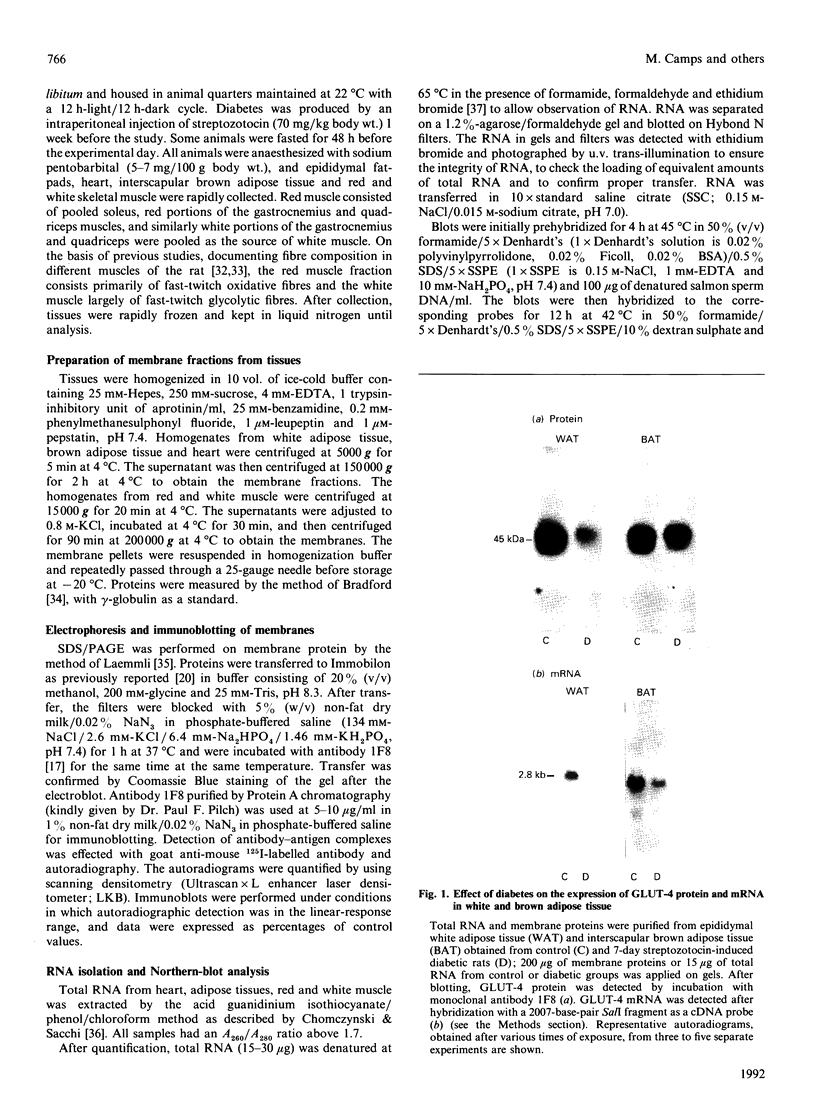
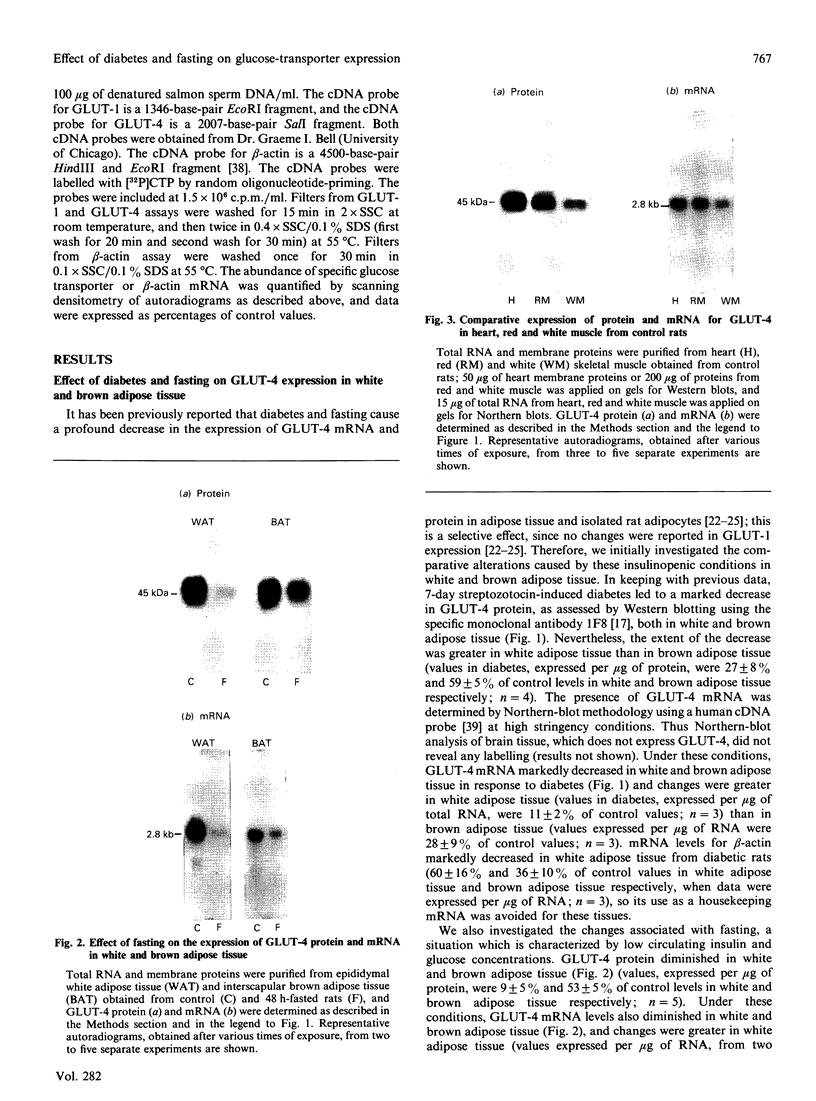
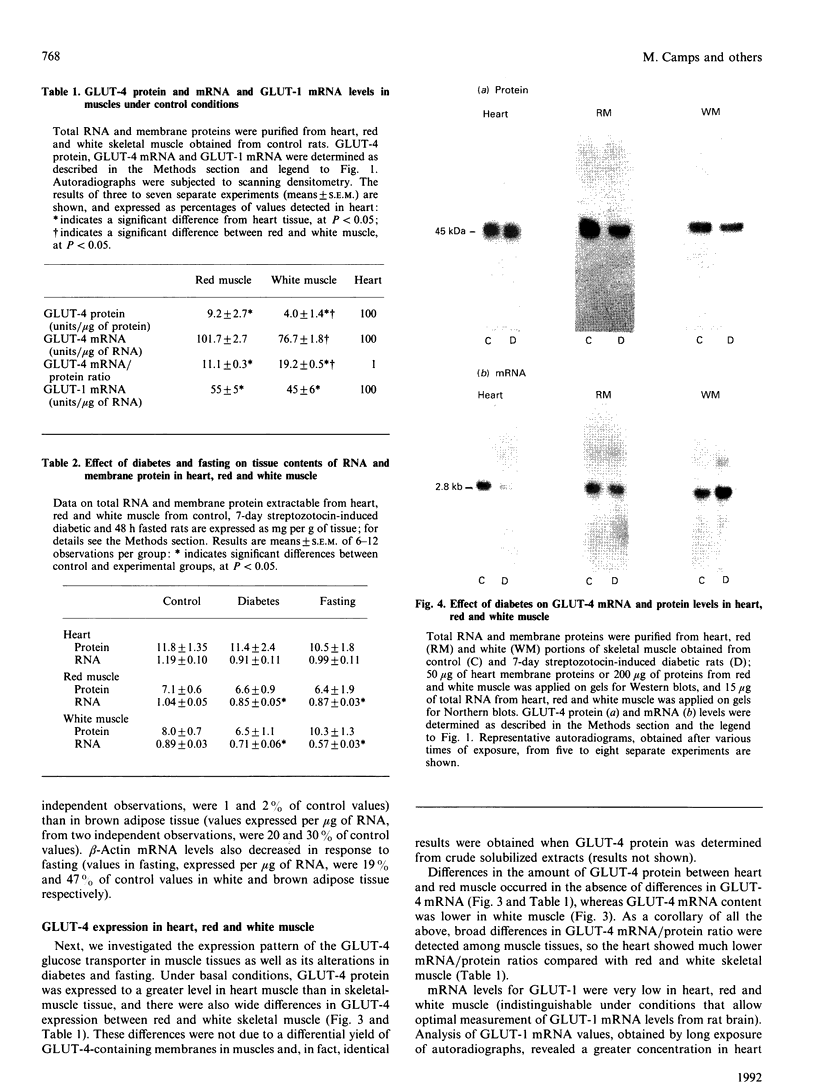
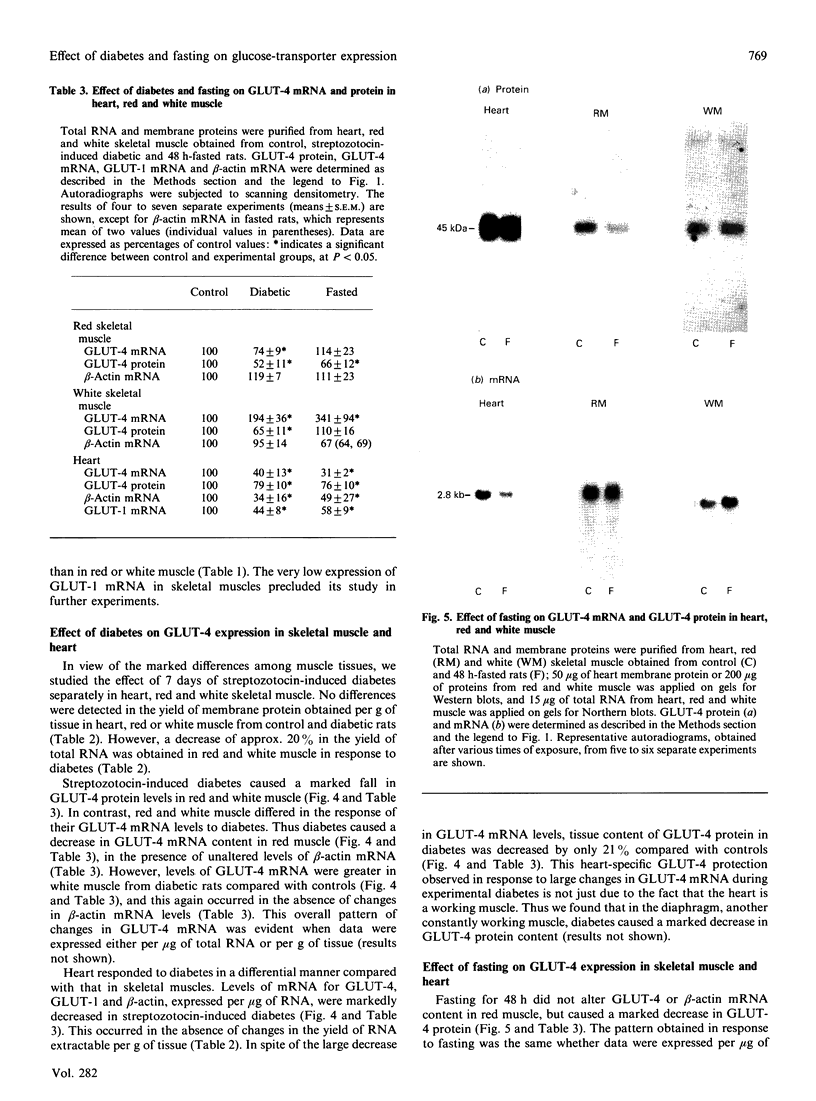



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Klinkerfuss G. H., Terjung R. L., Molé P. A., Holloszy J. O. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. 1972 Feb;222(2):373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- Berger J., Biswas C., Vicario P. P., Strout H. V., Saperstein R., Pilch P. F. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 1989 Jul 6;340(6228):70–72. doi: 10.1038/340070a0. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S. A., Goodman M. N., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Effects of starvation, diabetes, fatty acids, acetoacetate, insulin and exercise on glucose uptake and disposition. Biochem J. 1976 Aug 15;158(2):191–202. doi: 10.1042/bj1580191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourey R. E., Koranyi L., James D. E., Mueckler M., Permutt M. A. Effects of altered glucose homeostasis on glucose transporter expression in skeletal muscle of the rat. J Clin Invest. 1990 Aug;86(2):542–547. doi: 10.1172/JCI114742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Goodman M. N., Kalish F. N., Ruderman N. B. Insulin binding and sensitivity in rat skeletal muscle: effect of starvation. Am J Physiol. 1981 Feb;240(2):E184–E190. doi: 10.1152/ajpendo.1981.240.2.E184. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron M. J., Kahn B. B. Divergent molecular mechanisms for insulin-resistant glucose transport in muscle and adipose cells in vivo. J Biol Chem. 1990 May 15;265(14):7994–8000. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cusin I., Terrettaz J., Rohner-Jeanrenaud F., Zarjevski N., Assimacopoulos-Jeannet F., Jeanrenaud B. Hyperinsulinemia increases the amount of GLUT4 mRNA in white adipose tissue and decreases that of muscles: a clue for increased fat depot and insulin resistance. Endocrinology. 1990 Dec;127(6):3246–3248. doi: 10.1210/endo-127-6-3246. [DOI] [PubMed] [Google Scholar]
- Eckel J., Gerlach-Eskuchen E., Reinauer H. G-protein-mediated regulation of the insulin-responsive glucose transporter in isolated cardiac myocytes. Biochem J. 1990 Dec 15;272(3):691–696. doi: 10.1042/bj2720691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaim K. E., Copenhaver M. E., Jefferson L. S. Effects of diabetes on protein synthesis in fast- and slow-twitch rat skeletal muscle. Am J Physiol. 1980 Jul;239(1):E88–E95. doi: 10.1152/ajpendo.1980.239.1.E88. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M., McCall A. L., Lodish H. F. Distribution of glucose transporter messenger RNA transcripts in tissues of rat and man. J Clin Invest. 1987 Feb;79(2):657–661. doi: 10.1172/JCI112864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Birnbaum M. J. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science. 1989 Jul 7;245(4913):60–63. doi: 10.1126/science.2662408. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Ruderman N. B. Insulin sensitivity of rat skeletal muscle: effects of starvation and aging. Am J Physiol. 1979 May;236(5):E519–E523. doi: 10.1152/ajpendo.1979.236.5.E519. [DOI] [PubMed] [Google Scholar]
- Henriksen E. J., Bourey R. E., Rodnick K. J., Koranyi L., Permutt M. A., Holloszy J. O. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol. 1990 Oct;259(4 Pt 1):E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- James D. E., Jenkins A. B., Kraegen E. W. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol. 1985 May;248(5 Pt 1):E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- James D. E., Zorzano A., Böni-Schnetzler M., Nemenoff R. A., Powers A., Pilch P. F., Ruderman N. B. Intrinsic differences of insulin receptor kinase activity in red and white muscle. J Biol Chem. 1986 Nov 15;261(32):14939–14944. [PubMed] [Google Scholar]
- Jefferson L. S. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes. 1980 Jun;29(6):487–496. doi: 10.2337/diab.29.6.487. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Flores-Riveros J. R., McLenithan J. C., Janicot M., Lane M. D. Transcriptional repression of the mouse insulin-responsive glucose transporter (GLUT4) gene by cAMP. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1933–1937. doi: 10.1073/pnas.88.5.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Simpson I. A., Cushman S. W. Divergent mechanisms for the insulin resistant and hyperresponsive glucose transport in adipose cells from fasted and refed rats. Alterations in both glucose transporter number and intrinsic activity. J Clin Invest. 1988 Aug;82(2):691–699. doi: 10.1172/JCI113649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. A possible mechanism of insulin resistance in the rat adipose cell in streptozotocin-induced diabetes mellitus. Depletion of intracellular glucose transport systems. J Clin Invest. 1981 Sep;68(3):811–814. doi: 10.1172/JCI110318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern M., Wells J. A., Stephens J. M., Elton C. W., Friedman J. E., Tapscott E. B., Pekala P. H., Dohm G. L. Insulin responsiveness in skeletal muscle is determined by glucose transporter (Glut4) protein level. Biochem J. 1990 Sep 1;270(2):397–400. doi: 10.1042/bj2700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Bilan P. J., Cartee G. D., Gulve E. A., Holloszy J. O. Recruitment of GLUT-4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem Biophys Res Commun. 1990 Oct 30;172(2):728–736. doi: 10.1016/0006-291x(90)90735-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Effects of streptozotocin-induced diabetes on insulin binding, glucose transport, and intracellular glucose metabolism in isolated rat adipocytes. Diabetes. 1979 Feb;28(2):87–95. doi: 10.2337/diab.28.2.87. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Freychet P. Effect of fasting and streptozotocin diabetes on insulin binding and action in the isolated mouse soleus muscle. J Clin Invest. 1979 Nov;64(5):1505–1515. doi: 10.1172/JCI109609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE R. O. INFLUENCE OF FASTING AND REFEEDING ON RESPONSE OF ADIPOSE TISSUE TO INSULIN. Am J Physiol. 1963 Aug;205:222–224. doi: 10.1152/ajplegacy.1963.205.2.222. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Kasuga M., Kanazawa Y., Takaku F. Studies with antipeptide antibody suggest the presence of at least two types of glucose transporter in rat brain and adipocyte. J Biol Chem. 1988 Sep 15;263(26):13432–13439. [PubMed] [Google Scholar]
- Olefsky J. M. Effects of fasting on insulin binding, glucose transport, and glucose oxidation in isolated rat adipocytes: relationships between insulin receptors and insulin action. J Clin Invest. 1976 Dec;58(6):1450–1460. doi: 10.1172/JCI108601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter E. A., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982 Apr;69(4):785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz W. I., DeSautel S. L., Kayano T., Bell G. I., Pessin J. E. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature. 1989 Jul 6;340(6228):72–74. doi: 10.1038/340072a0. [DOI] [PubMed] [Google Scholar]
- Stirewalt W. S., Low R. B., Slaiby J. M. Insulin sensitivity and responsiveness of epitrochlearis and soleus muscles from fed and starved rats. Recognition of differential changes in insulin sensitivities of protein synthesis and glucose incorporation into glycogen. Biochem J. 1985 Apr 15;227(2):355–362. doi: 10.1042/bj2270355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strout H. V., Vicario P. P., Biswas C., Saperstein R., Brady E. J., Pilch P. F., Berger J. Vanadate treatment of streptozotocin diabetic rats restores expression of the insulin-responsive glucose transporter in skeletal muscle. Endocrinology. 1990 May;126(5):2728–2732. doi: 10.1210/endo-126-5-2728. [DOI] [PubMed] [Google Scholar]
- Vilaró S., Palacín M., Pilch P. F., Testar X., Zorzano A. Expression of an insulin-regulatable glucose carrier in muscle and fat endothelial cells. Nature. 1989 Dec 14;342(6251):798–800. doi: 10.1038/342798a0. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Holloszy J. O. Activation of glucose transport in diabetic muscle: responses to contraction and insulin. Am J Physiol. 1985 Sep;249(3 Pt 1):C233–C237. doi: 10.1152/ajpcell.1985.249.3.C233. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Zetan N., Henriksson J. Reversibility of decreased insulin-stimulated glucose transport capacity in diabetic muscle with in vitro incubation. Insulin is not required. J Biol Chem. 1987 Jun 5;262(16):7665–7671. [PubMed] [Google Scholar]
- Wang C. The D-glucose transporter is tissue-specific. Skeletal muscle and adipose tissue have a unique form of glucose transporter. J Biol Chem. 1987 Nov 15;262(32):15689–15695. [PubMed] [Google Scholar]
- Yamamoto T., Fukumoto H., Koh G., Yano H., Yasuda K., Masuda K., Ikeda H., Imura H., Seino Y. Liver and muscle-fat type glucose transporter gene expression in obese and diabetic rats. Biochem Biophys Res Commun. 1991 Mar 29;175(3):995–1002. doi: 10.1016/0006-291x(91)91663-w. [DOI] [PubMed] [Google Scholar]
- Zorzano A., Balon T. W., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle alpha-aminoisobutyric acid transport after exercise: enhanced stimulation by insulin. Am J Physiol. 1985 May;248(5 Pt 1):E546–E552. doi: 10.1152/ajpendo.1985.248.5.E546. [DOI] [PubMed] [Google Scholar]
- Zorzano A., James D. E., Ruderman N. B., Pilch P. F. Insulin-like growth factor I binding and receptor kinase in red and white muscle. FEBS Lett. 1988 Jul 18;234(2):257–262. doi: 10.1016/0014-5793(88)80093-0. [DOI] [PubMed] [Google Scholar]
- Zorzano A., Wilkinson W., Kotliar N., Thoidis G., Wadzinkski B. E., Ruoho A. E., Pilch P. F. Insulin-regulated glucose uptake in rat adipocytes is mediated by two transporter isoforms present in at least two vesicle populations. J Biol Chem. 1989 Jul 25;264(21):12358–12363. [PubMed] [Google Scholar]