Abstract
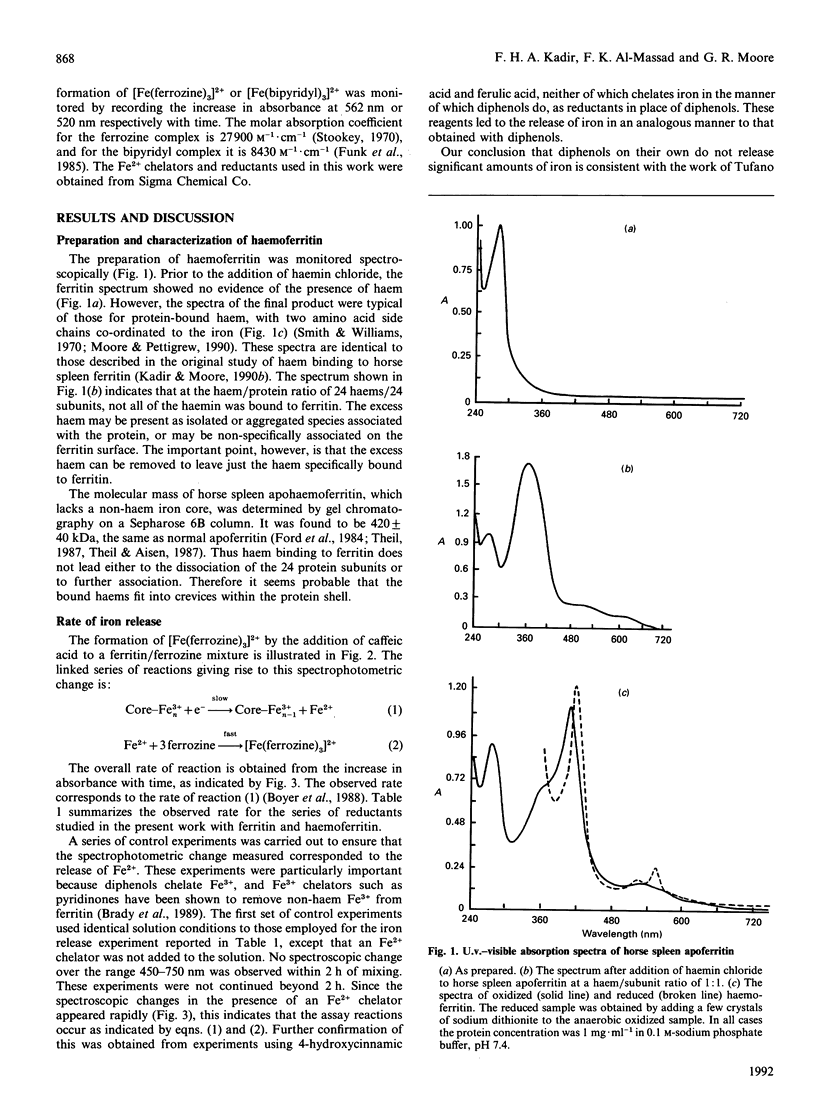
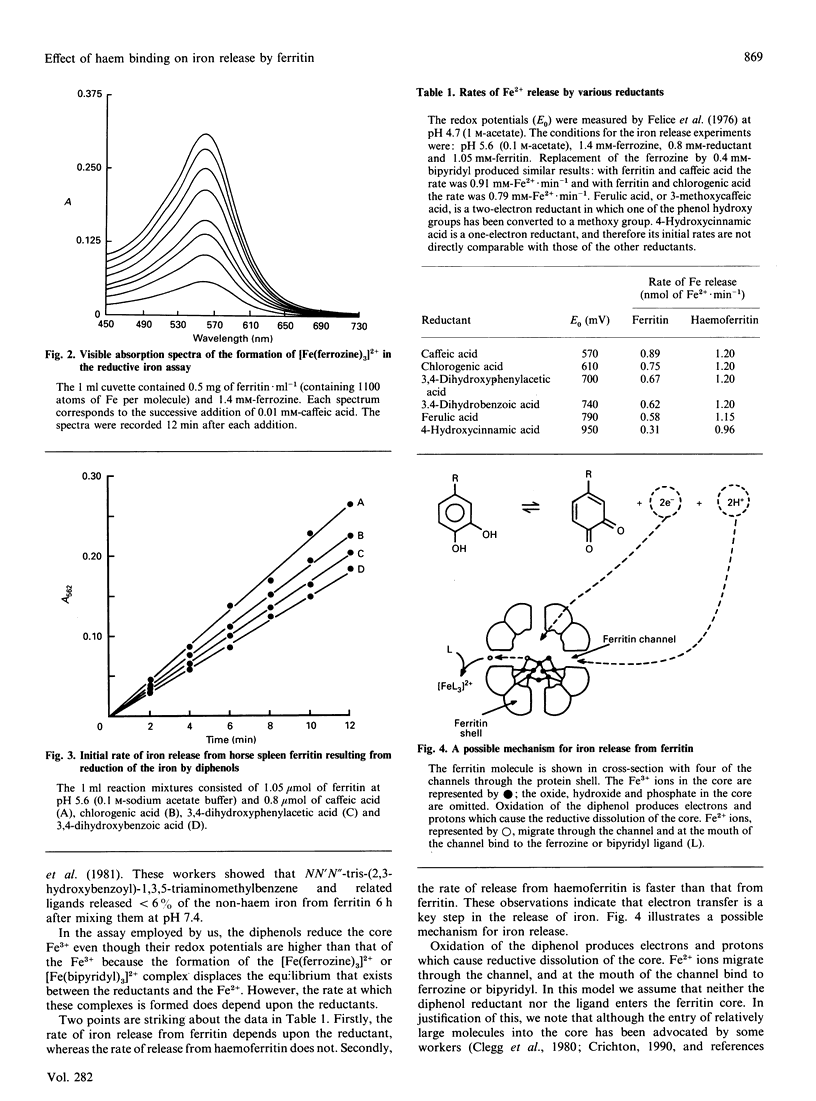
Horse spleen ferritin is shown to bind haem to generate a haemoprotein, named herein haemoferritin. A total of 14-16 haem molecules are bound per 24 subunits of ferritin. The molecular mass of the non-haem-iron-free haemoferritin has been determined to be 420 +/- 40 kDa, indicating that haem binding does not lead to dissociation of the 24 subunits that comprise the ferritin molecule. The functional role of the bound haem has been investigated with respect to the release of iron from the non-haem iron core. The bound haem is shown to increase the rate of iron release in a reductive assay system. In the absence of haem the rate of iron release depends on the redox potential of the reductant, but in the presence of haem the rate is largely independent of the reductant and is faster than the rate for the haem-free ferritin. These data haem, but in the presence of haem electron transfer is not rate-limiting.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer R. F., Clark H. M., LaRoche A. P. Reduction and release of ferritin iron by plant phenolics. J Inorg Biochem. 1988 Mar;32(3):171–181. doi: 10.1016/0162-0134(88)80025-4. [DOI] [PubMed] [Google Scholar]
- Brady M. C., Lilley K. S., Treffry A., Harrison P. M., Hider R. C., Taylor P. D. Release of iron from ferritin molecules and their iron-cores by 3-hydroxypyridinone chelators in vitro. J Inorg Biochem. 1989 Jan;35(1):9–22. doi: 10.1016/0162-0134(89)84002-4. [DOI] [PubMed] [Google Scholar]
- Clegg G. A., Fitton J. E., Harrison P. M., Treffry A. Ferritin: molecular structure and iron-storage mechanisms. Prog Biophys Mol Biol. 1980;36(2-3):56–86. [PubMed] [Google Scholar]
- Crichton R. R. Proteins of iron storage and transport. Adv Protein Chem. 1990;40:281–363. doi: 10.1016/s0065-3233(08)60288-0. [DOI] [PubMed] [Google Scholar]
- Felice L. J., King W. P., Kissinger P. T. A new liquid chromatography approach to plant phenolics. Application to the determination of chlorogenic acid in sunflower meal. J Agric Food Chem. 1976 Mar-Apr;24(2):380–382. doi: 10.1021/jf60204a042. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Funk F., Lenders J. P., Crichton R. R., Schneider W. Reductive mobilisation of ferritin iron. Eur J Biochem. 1985 Oct 1;152(1):167–172. doi: 10.1111/j.1432-1033.1985.tb09177.x. [DOI] [PubMed] [Google Scholar]
- Jacobs D. L., Watt G. D., Frankel R. B., Papaefthymiou G. C. Redox reactions associated with iron release from mammalian ferritin. Biochemistry. 1989 Feb 21;28(4):1650–1655. doi: 10.1021/bi00430a033. [DOI] [PubMed] [Google Scholar]
- Jones T., Spencer R., Walsh C. Mechanism and kinetics of iron release from ferritin by dihydroflavins and dihydroflavin analogues. Biochemistry. 1978 Sep 19;17(19):4011–4017. doi: 10.1021/bi00612a021. [DOI] [PubMed] [Google Scholar]
- Kadir F. H., Moore G. R. Bacterial ferritin contains 24 haem groups. FEBS Lett. 1990 Oct 1;271(1-2):141–143. doi: 10.1016/0014-5793(90)80391-u. [DOI] [PubMed] [Google Scholar]
- Kadir F. H., Moore G. R. Haem binding to horse spleen ferritin. FEBS Lett. 1990 Dec 10;276(1-2):81–84. doi: 10.1016/0014-5793(90)80512-h. [DOI] [PubMed] [Google Scholar]
- Mayo S. L., Ellis W. R., Jr, Crutchley R. J., Gray H. B. Long-range electron transfer in heme proteins. Science. 1986 Aug 29;233(4767):948–952. doi: 10.1126/science.3016897. [DOI] [PubMed] [Google Scholar]
- Stiefel E. I., Watt G. D. Azotobacter cytochrome b557.5 is a bacterioferritin. Nature. 1979 May 3;279(5708):81–83. doi: 10.1038/279081a0. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Tufano T. P., Pecoraro V. L., Raymond K. N. Ferric ion sequestering agents: kinetics of iron release from ferritin to catechoylamides. Biochim Biophys Acta. 1981 May 29;668(3):420–428. doi: 10.1016/0005-2795(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Watt G. D., Frankel R. B., Papaefthymiou G. C. Reduction of mammalian ferritin. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3640–3643. doi: 10.1073/pnas.82.11.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]


