Abstract
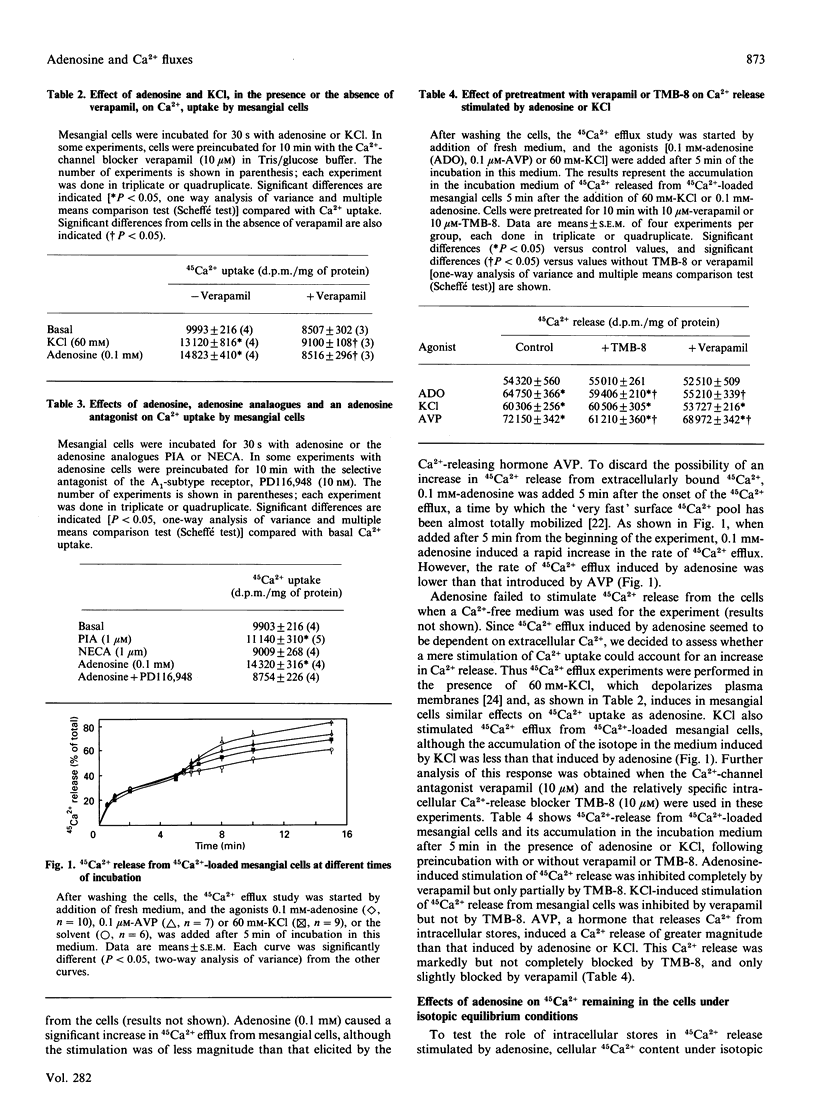
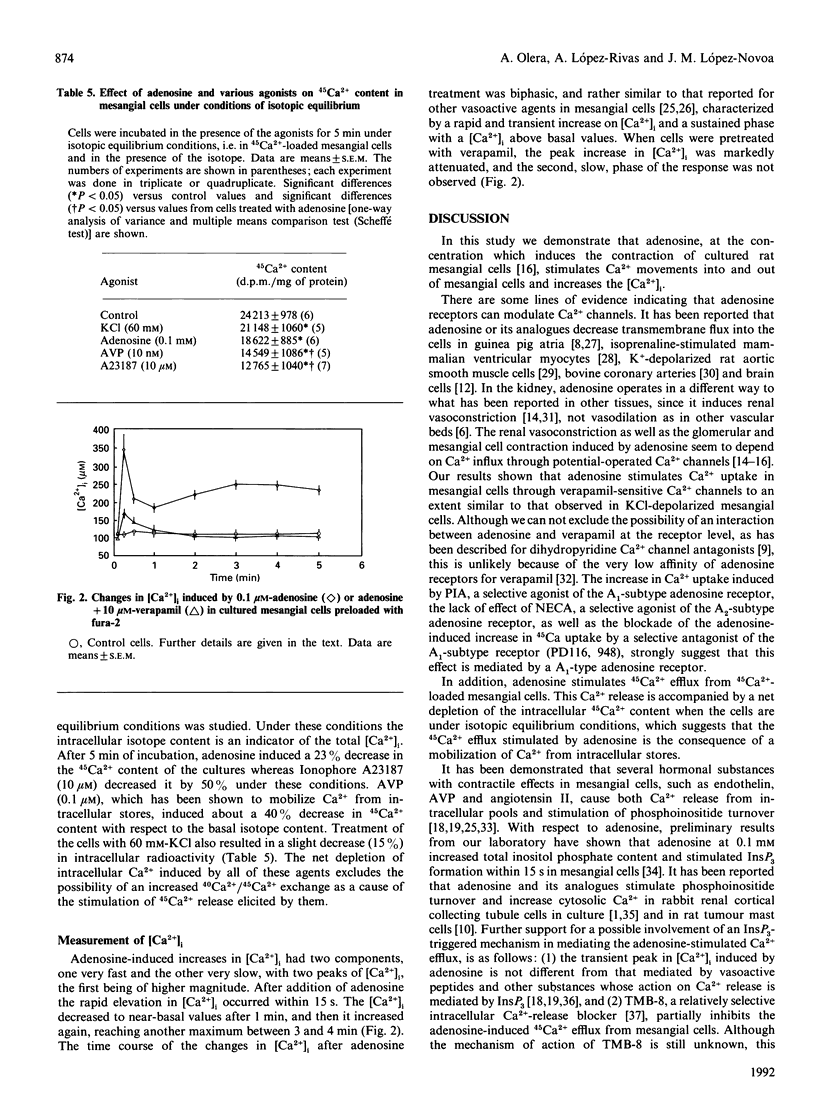
Adenosine has been associated with cellular Ca2+ metabolism in some cell types. Since adenosine is able to contract glomerular mesangial cells in culture, and since Ca2+ is the main messenger mediating contractile responses, we studied the effect of adenosine on 45Ca2+ movements into and out of mesangial cells and on the cytosolic free Ca2+ concentration ([Ca2+]i). Adenosine at 0.1 mM increased 45Ca2+ uptake (basal, 9993 +/- 216; + adenosine, 14823 +/- 410 d.p.m./mg; P less than 0.01) through verapamil-sensitive Ca2+ channels. These channels seem to be of the A1-adenosine receptor subtype. Adenosine also stimulated 45Ca2+ efflux from 45Ca(2+)-loaded mesangial cells. This effect was accompanied by a net depletion of intracellular 45Ca2+ content under isotopic equilibrium conditions (basal, 24213 +/- 978; + adenosine, 18622 +/- 885 d.p.m./mg; P less than 0.05). The increase in 45Ca2+ efflux was inhibited by a Ca(2+)-free medium or in the presence of 10 microM-verapamil. However, the intracellular Ca(2+)-release blocker TMB-8 (10 microM) only partially inhibited the adenosine-stimulated 45Ca2+ efflux. In addition, adenosine induced an elevation in [Ca2+]i in mesangial cells with an initial transient peak within 15 s (basal, 113 +/- 7; adenosine, 345 +/- 46 nM), and a secondary increase which was slower (3-4 min) and of lower magnitude than the initial peak (250 +/- 21 nM). In summary, adenosine elevates [Ca2+]i and stimulates both Ca2+ uptake from the extracellular pool and Ca2+ efflux from intracellular pools in mesangial cells. The Ca2+ release from internal stores is produced by a combination of a TMB-8-inhibitable and a non-TMB-8-inhibitable mechanism, and seems to be dependent on Ca2+ influx.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Cunha-Melo J. R., Saul W. F., Beaven M. A. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J Biol Chem. 1990 Jan 15;265(2):745–753. [PubMed] [Google Scholar]
- Arend L. J., Burnatowska-Hledin M. A., Spielman W. S. Adenosine receptor-mediated calcium mobilization in cortical collecting tubule cells. Am J Physiol. 1988 Nov;255(5 Pt 1):C581–C588. doi: 10.1152/ajpcell.1988.255.5.C581. [DOI] [PubMed] [Google Scholar]
- Arend L. J., Handler J. S., Rhim J. S., Gusovsky F., Spielman W. S. Adenosine-sensitive phosphoinositide turnover in a newly established renal cell line. Am J Physiol. 1989 Jun;256(6 Pt 2):F1067–F1074. doi: 10.1152/ajprenal.1989.256.6.F1067. [DOI] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Weber P. C., Gronich J. H. PAF and PDGF increase cytosolic [Ca2+] and phospholipase activity in mesangial cells. Am J Physiol. 1988 Jan;254(1 Pt 2):F87–F94. doi: 10.1152/ajprenal.1988.254.1.F87. [DOI] [PubMed] [Google Scholar]
- Borle A. B., Studer R. Effects of calcium ionophores on the transport and distribution of calcium in isolated cells and in liver and kidney slices. J Membr Biol. 1978 Jan 12;38(1-2):51–72. doi: 10.1007/BF01875162. [DOI] [PubMed] [Google Scholar]
- Brückner R., Fenner A., Meyer W., Nobis T. M., Schmitz W., Scholz H. Cardiac effects of adenosine and adenosine analogs in guinea-pig atrial and ventricular preparations: evidence against a role of cyclic AMP and cyclic GMP. J Pharmacol Exp Ther. 1985 Sep;234(3):766–774. [PubMed] [Google Scholar]
- Cerbai E., Klöckner U., Isenberg G. Ca-antagonistic effects of adenosine in guinea pig atrial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H872–H878. doi: 10.1152/ajpheart.1988.255.4.H872. [DOI] [PubMed] [Google Scholar]
- Cheung W. T., Shi M. M., Young J. D., Lee C. M. Inhibition of radioligand binding to A1 adenosine receptors by Bay K8644 and nifedipine. Biochem Pharmacol. 1987 Jul 1;36(13):2183–2186. doi: 10.1016/0006-2952(87)90148-1. [DOI] [PubMed] [Google Scholar]
- Chin J. H., Delorenzo R. J. Cobalt ion enhancement of 2-chloro[3H]adenosine binding to a novel class of adenosine receptors in brain: antagonism by calcium. Brain Res. 1985 Dec 2;348(2):381–386. doi: 10.1016/0006-8993(85)90462-7. [DOI] [PubMed] [Google Scholar]
- Chiou C. Y., Malagodi M. H. Studies on the mechanism of action of a new Ca-2+ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride in smooth and skeletal muscles. Br J Pharmacol. 1975 Feb;53(2):279–285. doi: 10.1111/j.1476-5381.1975.tb07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. C., Bidani A. Renal effects of selective adenosine receptor agonists in anesthetized rats. Am J Physiol. 1987 Feb;252(2 Pt 2):F299–F303. doi: 10.1152/ajprenal.1987.252.2.F299. [DOI] [PubMed] [Google Scholar]
- De Biasi M., Froldi G., Ragazzi E., Pandolfo L., Caparrotta L., Fassina G. Potassium channel blockers differentially affect carbachol and (-)-N6-phenylisopropyladenosine on guinea-pig atria. Br J Pharmacol. 1989 Jul;97(3):866–872. doi: 10.1111/j.1476-5381.1989.tb12026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahunty T. M., Cronin M. J., Linden J. Regulation of GH3-cell function via adenosine A1 receptors. Inhibition of prolactin release, cyclic AMP production and inositol phosphate generation. Biochem J. 1988 Oct 1;255(1):69–77. doi: 10.1042/bj2550069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. Insulin-dependent contractility of glomerular mesangial cells in response to angiotensin II, platelet-activating factor and endothelin is attenuated by prostaglandin E2. Biochem J. 1990 Dec 15;272(3):561–568. doi: 10.1042/bj2720561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Malbon C. C. Regulation of adenylate cyclase by adenosine. Mol Cell Biochem. 1979 Jun 15;25(3):143–169. doi: 10.1007/BF00235364. [DOI] [PubMed] [Google Scholar]
- Farese R. V. Calcium as an intracellular mediator of hormone action: intracellular phospholipid signaling systems. Am J Med Sci. 1988 Oct;296(4):223–230. [PubMed] [Google Scholar]
- Fenton R. A., Bruttig S. P., Rubio R., Berne R. M. Effect of adenosine on calcium uptake by intact and cultured vascular smooth muscle. Am J Physiol. 1982 May;242(5):H797–H804. doi: 10.1152/ajpheart.1982.242.5.H797. [DOI] [PubMed] [Google Scholar]
- Fuse I., Tai H. H. Does protein kinase C activation mediate thrombin-induced arachidonate release in human platelets? Biochim Biophys Acta. 1988 Oct 28;972(1):54–59. doi: 10.1016/0167-4889(88)90102-4. [DOI] [PubMed] [Google Scholar]
- Ganz M. B., Pekar S. K., Perfetto M. C., Sterzel R. B. Arginine vasopressin promotes growth of rat glomerular mesangial cells in culture. Am J Physiol. 1988 Nov;255(5 Pt 2):F898–F906. doi: 10.1152/ajprenal.1988.255.5.F898. [DOI] [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hassid A., Pidikiti N., Gamero D. Effects of vasoactive peptides on cytosolic calcium in cultured mesangial cells. Am J Physiol. 1986 Dec;251(6 Pt 2):F1018–F1028. doi: 10.1152/ajprenal.1986.251.6.F1018. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Belardinelli L. Ionic basis for the antagonism between adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res. 1984 Sep;55(3):309–325. doi: 10.1161/01.res.55.3.309. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rivas A., Rozengurt E. Serum rapidly mobilizes calcium from an intracellular pool in quiescent fibroblastic cells. Biochem Biophys Res Commun. 1983 Jul 18;114(1):240–247. doi: 10.1016/0006-291x(83)91619-4. [DOI] [PubMed] [Google Scholar]
- Macias-Nuñez J. F., García-Iglesias C., Santos J. C., Sanz E., López-Novoa J. M. Influence of plasma renin content, intrarenal angiotensin II, captopril, and calcium channel blockers on the vasoconstriction and renin release promoted by adenosine in the kidney. J Lab Clin Med. 1985 Nov;106(5):562–567. [PubMed] [Google Scholar]
- Marquardt D. L., Walker L. L. Alteration of mast cell responsiveness to adenosine by pertussis toxin. Biochem Pharmacol. 1988 Oct 15;37(20):4019–4025. doi: 10.1016/0006-2952(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Martonosi A. N. Mechanisms of Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Physiol Rev. 1984 Oct;64(4):1240–1320. doi: 10.1152/physrev.1984.64.4.1240. [DOI] [PubMed] [Google Scholar]
- Mené P., Simonson M. S., Dunn M. J. Physiology of the mesangial cell. Physiol Rev. 1989 Oct;69(4):1347–1424. doi: 10.1152/physrev.1989.69.4.1347. [DOI] [PubMed] [Google Scholar]
- Olivera A., Lamas S., Rodriguez-Puyol D., López-Novoa J. M. Adenosine induces mesangial cell contraction by an A1-type receptor. Kidney Int. 1989 Jun;35(6):1300–1305. doi: 10.1038/ki.1989.126. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Cross-talk between transmembrane signalling systems: a prerequisite for the delicate regulation of glomerular haemodynamics by mesangial cells. Eur J Clin Invest. 1989 Aug;19(4):347–361. doi: 10.1111/j.1365-2362.1989.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Extracellular ATP stimulates polyphosphoinositide hydrolysis and prostaglandin synthesis in rat renal mesangial cells. Involvement of a pertussis toxin-sensitive guanine nucleotide binding protein and feedback inhibition by protein kinase C. Cell Signal. 1990;2(2):129–138. doi: 10.1016/0898-6568(90)90016-4. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Renard D., Petit-Koskas E., Génot E., Dugas B., Poggioli J., Kolb J. P. Activation of the phosphatidylinositol metabolic pathway by low molecular weight B cell growth factor. Eur J Immunol. 1988 Nov;18(11):1705–1711. doi: 10.1002/eji.1830181108. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A., Sebastião A. M. Adenosine receptors and calcium: basis for proposing a third (A3) adenosine receptor. Prog Neurobiol. 1986;26(3):179–209. doi: 10.1016/0301-0082(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Puyol D., Lamas S., Olivera A., López-Farré A., Ortega G., Hernando L., López-Novoa J. M. Actions of cyclosporin A on cultured rat mesangial cells. Kidney Int. 1989 Feb;35(2):632–637. doi: 10.1038/ki.1989.32. [DOI] [PubMed] [Google Scholar]
- Rossi N., Churchill P., Ellis V., Amore B. Mechanism of adenosine receptor-induced renal vasoconstriction in rats. Am J Physiol. 1988 Oct;255(4 Pt 2):H885–H890. doi: 10.1152/ajpheart.1988.255.4.H885. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Wann S., Mené P., Dubyak G. R., Kester M., Nakazato Y., Sedor J. R., Dunn M. J. Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J Clin Invest. 1989 Feb;83(2):708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakui M., Osipchuk Y. V., Petersen O. H. Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2(+)-induced Ca2+ release. Cell. 1990 Nov 30;63(5):1025–1032. doi: 10.1016/0092-8674(90)90505-9. [DOI] [PubMed] [Google Scholar]
- Yu Y. M., Lermioglu F., Hassid A. Modulation of Ca by agents affecting voltage-sensitive Ca channels in mesangial cells. Am J Physiol. 1989 Dec;257(6 Pt 2):F1094–F1099. doi: 10.1152/ajprenal.1989.257.6.F1094. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


