Abstract
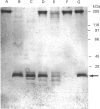
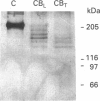
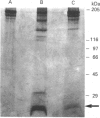
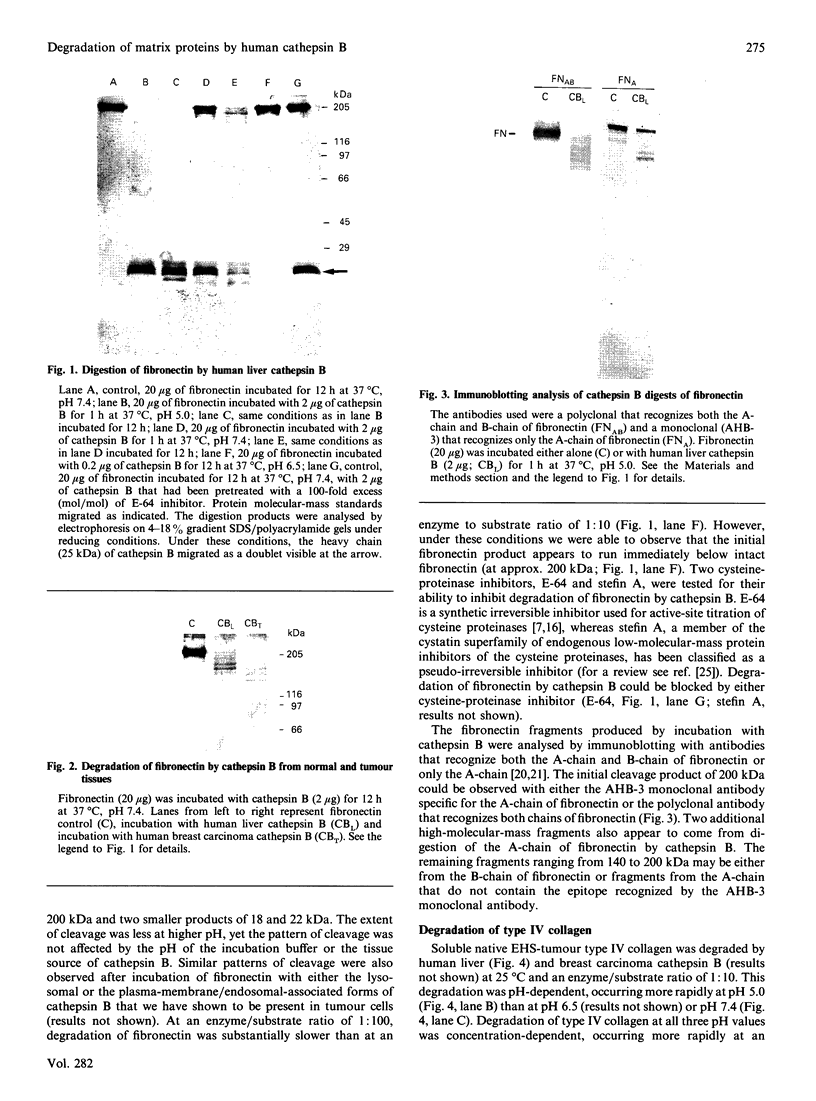
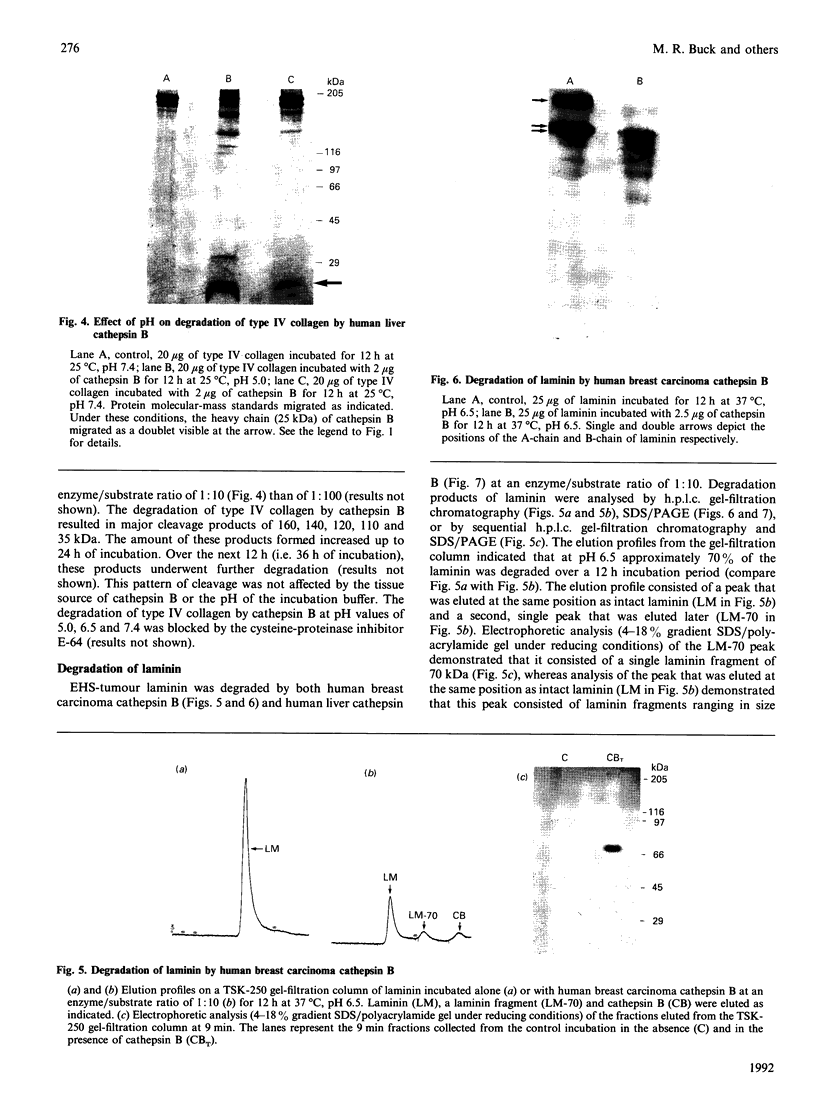
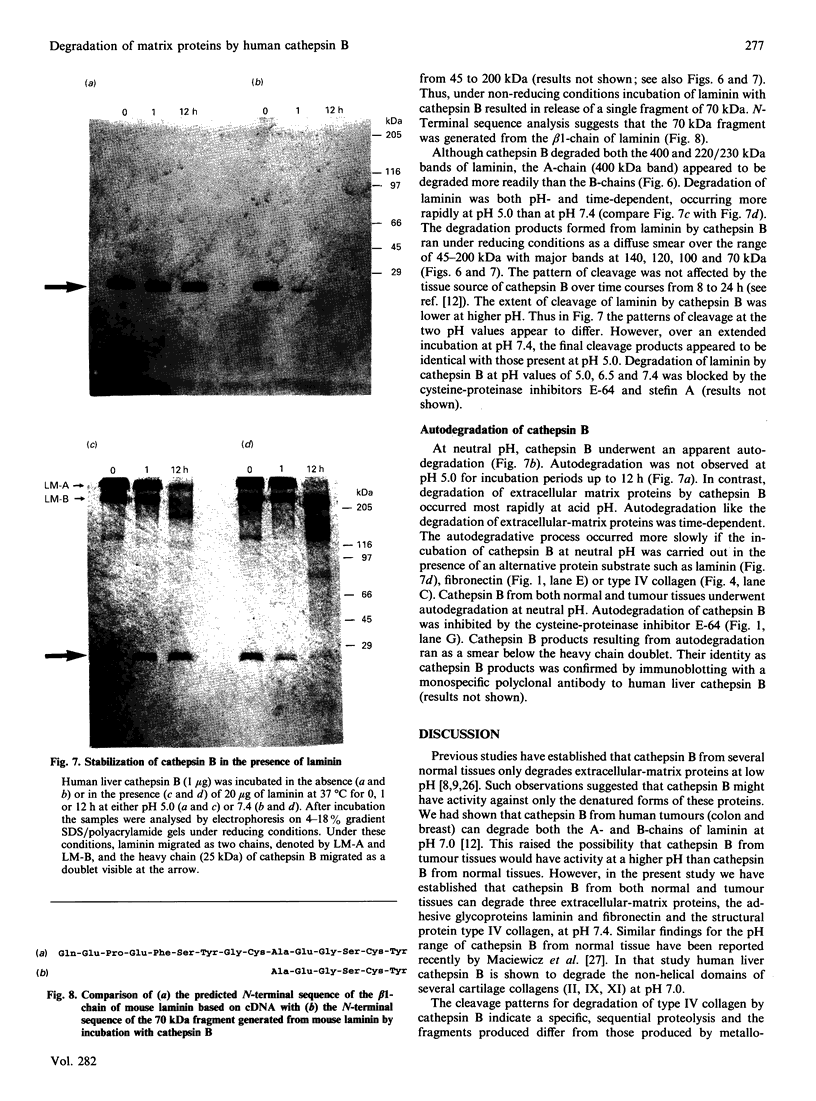
Our laboratory has previously demonstrated that increased malignancy of several histological types of human and animal tumours is associated with increases in their cathepsin B activity, particularly cathepsin B activity associated with plasma-membrane/endosomal vesicles or shed vesicles. Here we report that cathepsin B from normal or tumour tissues degrades purified extracellular-matrix components, type IV collagen, laminin and fibronectin, at both acid pH and neutral pH. The number and sizes of degradation products were analysed by SDS/PAGE. Cathepsin B from both sources exhibited similar activities towards, and similar patterns of cleavage of, the extracellular-matrix proteins. At neutral pH, cathepsin B from both sources appeared to undergo autodegradation, a process that was decreased in the presence of alternative substrates such as the extracellular-matrix proteins. Cathepsin B readily degraded type IV collagen at 25 degrees C, indicating activity towards native type IV collagen. Fibronectin degradation products of 100-200 kDa and of 18 and 22 kDa were observed. A single 70 kDa fragment was released from laminin under non-reducing conditions and multiple fragments ranging from 45 to 200 kDa under reducing conditions. These results suggest that cathepsin B at or near the surface of malignant tumour cells may play a functional role in the focal dissolution of extracellular matrices.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burleigh M. C., Barrett A. J., Lazarus G. S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J. 1974 Feb;137(2):387–398. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelberg M. K., McCarthy J. B., Skubitz A. P., Furcht L. T., Tsilibary E. C. Characterization of a synthetic peptide from type IV collagen that promotes melanoma cell adhesion, spreading, and motility. J Cell Biol. 1990 Jul;111(1):261–270. doi: 10.1083/jcb.111.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P., Roop D. R., Cheng C. K., Krieg T. M., Yuspa S. H. Molecular cloning of mouse epidermal cystatin A and detection of regulated expression in differentiation and tumorigenesis. Mol Carcinog. 1988;1(3):202–211. doi: 10.1002/mc.2940010309. [DOI] [PubMed] [Google Scholar]
- Khokha R., Waterhouse P., Yagel S., Lala P. K., Overall C. M., Norton G., Denhardt D. T. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989 Feb 17;243(4893):947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lah T. T., Buck M. R., Honn K. V., Crissman J. D., Rao N. C., Liotta L. A., Sloane B. F. Degradation of laminin by human tumor cathepsin B. Clin Exp Metastasis. 1989 Jul-Aug;7(4):461–468. doi: 10.1007/BF01753666. [DOI] [PubMed] [Google Scholar]
- Maciewicz R. A., Wotton S. F., Etherington D. J., Duance V. C. Susceptibility of the cartilage collagens types II, IX and XI to degradation by the cysteine proteinases, cathepsins B and L. FEBS Lett. 1990 Aug 20;269(1):189–193. doi: 10.1016/0014-5793(90)81151-d. [DOI] [PubMed] [Google Scholar]
- Mason R. W. Interaction of lysosomal cysteine proteinases with alpha 2-macroglobulin: conclusive evidence for the endopeptidase activities of cathepsins B and H. Arch Biochem Biophys. 1989 Sep;273(2):367–374. doi: 10.1016/0003-9861(89)90495-5. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Chelberg M. K., Mickelson D. J., Furcht L. T. Localization and chemical synthesis of fibronectin peptides with melanoma adhesion and heparin binding activities. Biochemistry. 1988 Feb 23;27(4):1380–1388. doi: 10.1021/bi00404a044. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Furcht L. T. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J Cell Biol. 1984 Apr;98(4):1474–1480. doi: 10.1083/jcb.98.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Morrison R. I., Barrett A. J., Dingle J. T., Prior D. Cathepsins BI and D. Action on human cartilage proteoglycans. Biochim Biophys Acta. 1973 Apr 12;302(2):411–419. doi: 10.1016/0005-2744(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Palm S. L., McCarthy J. B., Furcht L. T. Alternative model for the internal structure of laminin. Biochemistry. 1985 Dec 17;24(26):7753–7760. doi: 10.1021/bi00347a038. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Margulies I. M., Goldfarb R. H., Madri J. A., Woodley D. T., Liotta L. A. Differential proteolytic susceptibility of laminin alpha and beta subunits. Arch Biochem Biophys. 1982 Nov;219(1):65–70. doi: 10.1016/0003-9861(82)90134-5. [DOI] [PubMed] [Google Scholar]
- Recklies A. D. Biochemical aspects of cellular migration and invasion. Biorheology. 1987;24(2):93–103. doi: 10.3233/bir-1987-24203. [DOI] [PubMed] [Google Scholar]
- Rich D. H., Brown M. A., Barrett A. J. Purification of cathepsin B by a new form of affinity chromatography. Biochem J. 1986 May 1;235(3):731–734. doi: 10.1042/bj2350731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhin J., Robinson D., Stevens M. A., Lah T. T., Honn K. V., Ryan R. E., Sloane B. F. Properties of a plasma membrane-associated cathepsin B-like cysteine proteinase in metastatic B16 melanoma variants. Cancer Res. 1987 Dec 15;47(24 Pt 1):6620–6628. [PubMed] [Google Scholar]
- Salo T., Liotta L. A., Tryggvason K. Purification and characterization of a murine basement membrane collagen-degrading enzyme secreted by metastatic tumor cells. J Biol Chem. 1983 Mar 10;258(5):3058–3063. [PubMed] [Google Scholar]
- Sloane B. F. Cathepsin B and cystatins: evidence for a role in cancer progression. Semin Cancer Biol. 1990 Apr;1(2):137–152. [PubMed] [Google Scholar]
- Sloane B. F., Moin K., Krepela E., Rozhin J. Cathepsin B and its endogenous inhibitors: the role in tumor malignancy. Cancer Metastasis Rev. 1990 Dec;9(4):333–352. doi: 10.1007/BF00049523. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Rozhin J., Hatfield J. S., Crissman J. D., Honn K. V. Plasma membrane-associated cysteine proteinases in human and animal tumors. Exp Cell Biol. 1987;55(4):209–224. doi: 10.1159/000163420. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Dehdarani A. H., Yonezawa S., Tang J. Porcine spleen cathepsin B is an exopeptidase. J Biol Chem. 1986 Jul 15;261(20):9375–9381. [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Thomas G. J., Davies M. The potential role of human kidney cortex cysteine proteinases in glomerular basement membrane degradation. Biochim Biophys Acta. 1989 Mar 24;990(3):246–253. doi: 10.1016/s0304-4165(89)80041-8. [DOI] [PubMed] [Google Scholar]
- Towatari T., Kawabata Y., Katunuma N. Crystallization and properties of cathepsin B from rat liver. Eur J Biochem. 1979 Dec;102(1):279–289. doi: 10.1111/j.1432-1033.1979.tb06290.x. [DOI] [PubMed] [Google Scholar]
- Tressel T., McCarthy J. B., Calaycay J., Lee T. D., Legesse K., Shively J. E., Pande H. Human plasma fibronectin. Demonstration of structural differences between the A- and B-chains in the III CS region. Biochem J. 1991 Mar 15;274(Pt 3):731–738. doi: 10.1042/bj2740731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Preparation of cathepsins B and H by covalent chromatography and characterization of their catalytic sites by reaction with a thiol-specific two-protonic-state reactivity probe. Kinetic study of cathepsins B and H extending into alkaline media and a rapid spectroscopic titration of cathepsin H at pH 3-4. Biochem J. 1985 Apr 15;227(2):511–519. doi: 10.1042/bj2270511. [DOI] [PMC free article] [PubMed] [Google Scholar]