Abstract
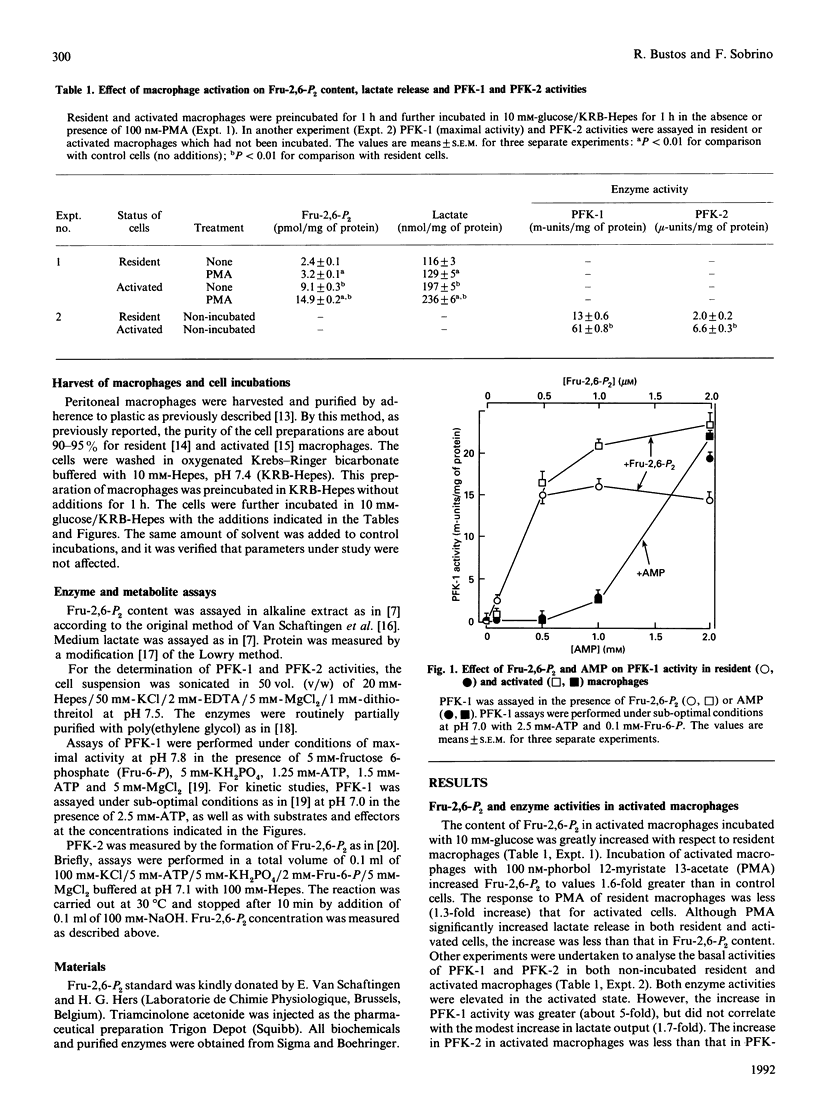
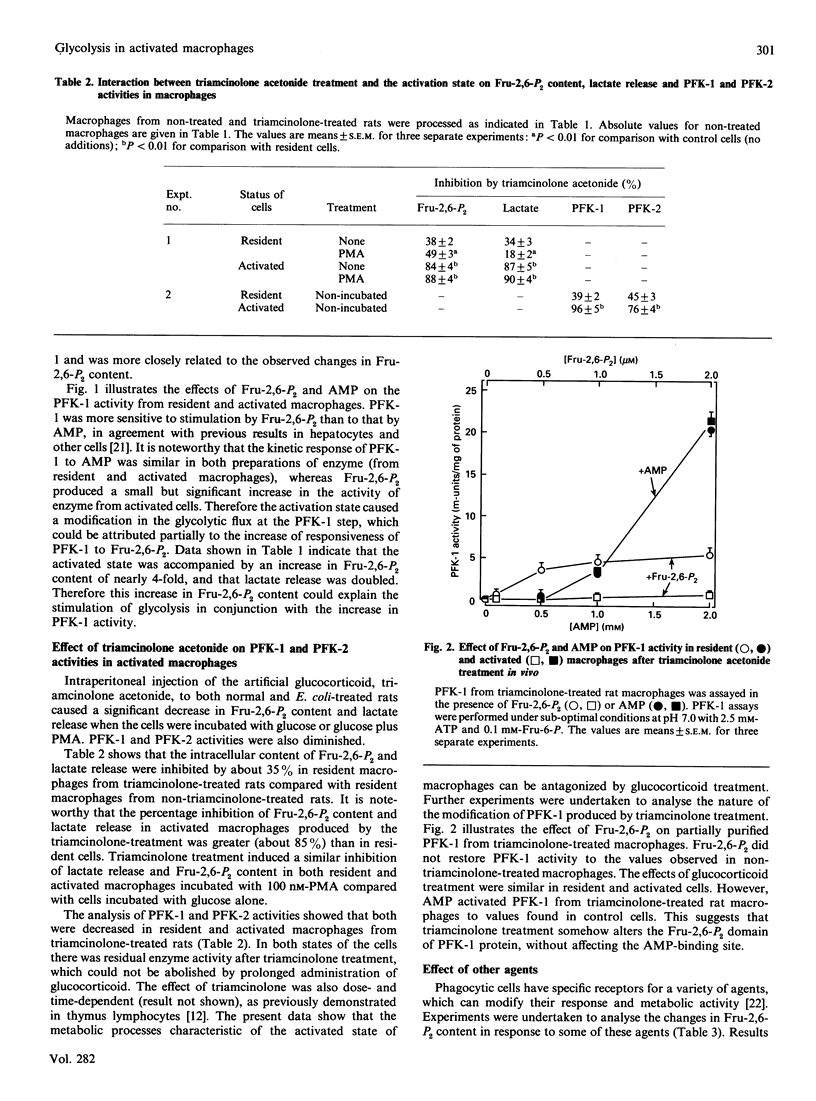
1. Peritoneal macrophages were prepared from control, Escherichia coli-treated and triamcinolone acetonide-treated rats. Control and E. coli-treated rats produced resident and activated macrophages respectively. Glycolysis in these cells was studied by the fructose 2,6-bisphosphate (Fru-2,6-P2) content, lactate release and 6-phosphofructo-1-kinase (PFK-1) and 6-phosphofructo-2-kinase (PFK-2) activities. 2. In activated macrophages, lactate release and Fru-2,6-P2 content were increased several-fold compared with those in resident cells. Moreover, the response of these parameters to phorbol 12-myristate 13-acetate in activated macrophages was greater than for resident cells. 3. PFK-2 activity was moderately increased (about 3-fold), but PFK-1 activity was increased 5-fold in activated macrophages compared with resident cells. Partially purified preparations of PFK-1 were sensitive to Fru-2,6-P2, with K0.5 about 0.25 microM in both control and activated cells. However, the Vmax. of PFK-1 from activated cells was increased. In addition, AMP stimulated PFK-1, but the kinetic pattern was different from that described for Fru-2,6-P2. Moreover there was no difference in the stimulation by AMP of PFK-1 from resident and activated cells. 4. Fru-2,6-P2 content and lactate release in macrophages from triamcinolone acetonide-treated rats were decreased in both resident and activated cells. Also, the glucocorticoid inhibited PFK-1 and PFK-2 activities in both resident and activated macrophages. PFK-1 from triamcinolone acetonide-treated rats was not stimulated by Fru-2,6-P2, whereas the effect of AMP was unchanged. The effects of glucocorticoid seem to be specific for phagocytic cells, since the glucocorticoid treatment increased PFK-1 and PFK-2 activities in liver.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Massoni R. J. Characteristics of bacterial lipopolysaccharide induction of interleukin 1 synthesis and secretion by human monocytes. Clin Exp Immunol. 1986 Jun;64(3):656–664. [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G. Glucocorticoid hormone action: an overview. Monogr Endocrinol. 1979;12:1–24. doi: 10.1007/978-3-642-81265-1_1. [DOI] [PubMed] [Google Scholar]
- Bosca L., Mojena M., Diaz-Guerra J. M., Marquez C. Phorbol 12,13-dibutyrate and mitogens increase fructose 2,6-bisphosphate in lymphocytes. Comparison of lymphocyte and rat-liver 6-phosphofructo-2-kinase. Eur J Biochem. 1988 Aug 1;175(2):317–323. doi: 10.1111/j.1432-1033.1988.tb14199.x. [DOI] [PubMed] [Google Scholar]
- Bustos R., Sobrino F. Control of fructose 2,6-bisphosphate levels in rat macrophages by glucose and phorbol ester. FEBS Lett. 1989 Jul 17;251(1-2):143–146. doi: 10.1016/0014-5793(89)81444-9. [DOI] [PubMed] [Google Scholar]
- Chiara M. D., Bedoya F., Sobrino F. Cyclosporin A inhibits phorbol ester-induced activation of superoxide production in resident mouse peritoneal macrophages. Biochem J. 1989 Nov 15;264(1):21–26. doi: 10.1042/bj2640021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel C. W., Halkias D. G., Klein T. W., Szentivanyi A. Characteristics of cells present in peritoneal fluids of mice injected intraperitoneally with Bordetella pertussis. Infect Immun. 1976 Jan;13(1):263–272. doi: 10.1128/iai.13.1.263-272.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol. 1979 Oct;123(4):1624–1631. [PubMed] [Google Scholar]
- Gualberto A., Molinero P., Sobrino F. The effect of experimental hypothyroidism on phosphofructokinase activity and fructose 2,6-bisphosphate concentrations in rat heart. Biochem J. 1987 May 15;244(1):137–142. doi: 10.1042/bj2440137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Blackmore P. F., Shikama H., Robinson-Steiner A., Exton J. H. Regulation of fructose-2,6-bisphosphate content in rat hepatocytes, perfused hearts, and perfused hindlimbs. J Biol Chem. 1982 Apr 25;257(8):4308–4313. [PubMed] [Google Scholar]
- Hue L. Role of fructose 2,6-bisphosphate in the stimulation of glycolysis by anoxia in isolated hepatocytes. Biochem J. 1982 Aug 15;206(2):359–365. doi: 10.1042/bj2060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Sobrino F., Bosca L. Difference in glucose sensitivity of liver glycolysis and glycogen synthesis. Relationship between lactate production and fructose 2,6-bisphosphate concentration. Biochem J. 1984 Dec 15;224(3):779–786. doi: 10.1042/bj2240779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Lefkowitz D. L., Hsieh T. C., Mills K., Castro A. Induction of tumor necrosis factor and cytotoxicity by macrophages exposed to lactoperoxidase and microperoxidase. Life Sci. 1990;47(8):703–709. doi: 10.1016/0024-3205(90)90625-2. [DOI] [PubMed] [Google Scholar]
- Marker A. J., Colosia A. D., Tauler A., Solomon D. H., Cayre Y., Lange A. J., el-Maghrabi M. R., Pilkis S. J. Glucocorticoid regulation of hepatic 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene expression. J Biol Chem. 1989 Apr 25;264(12):7000–7004. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Martín F., Gualberto A., Sobrino F., Pintado E. Thimerosal induces calcium mobilization, fructose 2,6-bisphosphate synthesis and cytoplasmic alkalinization in rat thymus lymphocytes. Biochim Biophys Acta. 1991 Jan 10;1091(1):110–114. doi: 10.1016/0167-4889(91)90229-q. [DOI] [PubMed] [Google Scholar]
- McCall C. E., Volk J. V., Cooper M. R., DeChatelet L. R. Effect of adrenocorticosteroid on glucose metabolism in BCG-sensitized alveolar macrophages. Infect Immun. 1971 Sep;4(3):315–317. doi: 10.1128/iai.4.3.315-317.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Aurioles V. R., Sobrino F. Glucocorticoids inhibit fructose 2,6-bisphosphate synthesis in rat thymocytes. Opposite effect of cycloheximide. Biochim Biophys Acta. 1991 Jan 10;1091(1):96–100. doi: 10.1016/0167-4889(91)90227-o. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. Separation of macrophages on plastic and glass surfaces. Methods Enzymol. 1984;108:294–297. doi: 10.1016/s0076-6879(84)08094-0. [DOI] [PubMed] [Google Scholar]
- Newsholme P., Curi R., Gordon S., Newsholme E. A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J. 1986 Oct 1;239(1):121–125. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P., Newsholme E. A. Rates of utilization of glucose, glutamine and oleate and formation of end-products by mouse peritoneal macrophages in culture. Biochem J. 1989 Jul 1;261(1):211–218. doi: 10.1042/bj2610211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Norton J. M., Munck A. In vitro actions of glucocorticoids on murine macrophages: effects on glucose transport and metabolism, growth in culture, and protein synthesis. J Immunol. 1980 Jul;125(1):259–266. [PubMed] [Google Scholar]
- Ralph P., Ito M., Broxmeyer H. E., Nakoinz I. Corticosteroids block newly induced but not constitutive functions of macrophage cell lines: myeloid colony-stimulating activity production, latex phagocytosis, and antibody-dependent lysis of RBC and tumor targets. J Immunol. 1978 Jul;121(1):300–303. [PubMed] [Google Scholar]
- Rider M. H., Hue L. Regulation of fructose 2,6-bisphosphate concentration in white adipose tissue. Biochem J. 1985 Jan 15;225(2):421–428. doi: 10.1042/bj2250421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Sobrino F., Gualberto A., González-Rivero J. Regulation of fructose-2,6-bisphosphate and glycogen synthesis by dichloroacetate and phenazine methosulphate in rat adipose tissue. Biochem Int. 1986 May;12(5):767–774. [PubMed] [Google Scholar]
- Sobrino F., Gualberto A. Hormonal regulation of fructose 2,6-bisphosphate levels in epididymal adipose tissue of rat. FEBS Lett. 1985 Mar 25;182(2):327–330. doi: 10.1016/0014-5793(85)80326-4. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Evanson J. M., Woolley D. E. Comparative effects of interleukin 1 and a phorbol ester on rheumatoid synovial cell fructose 2,6-bisphosphate content and prostaglandin E production. Biochem Biophys Res Commun. 1988 Jan 15;150(1):349–354. doi: 10.1016/0006-291x(88)90527-x. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Enzymatic basis of macrophage activation. Kinetic analysis of superoxide production in lysates of resident and activated mouse peritoneal macrophages and granulocytes. J Biol Chem. 1984 Apr 10;259(7):4305–4312. [PubMed] [Google Scholar]
- Van Schaftingen E., Jett M. F., Hue L., Hers H. G. Control of liver 6-phosphofructokinase by fructose 2,6-bisphosphate and other effectors. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3483–3486. doi: 10.1073/pnas.78.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]


