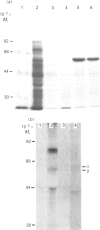
Abstract
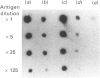
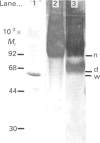
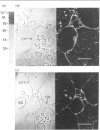
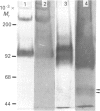
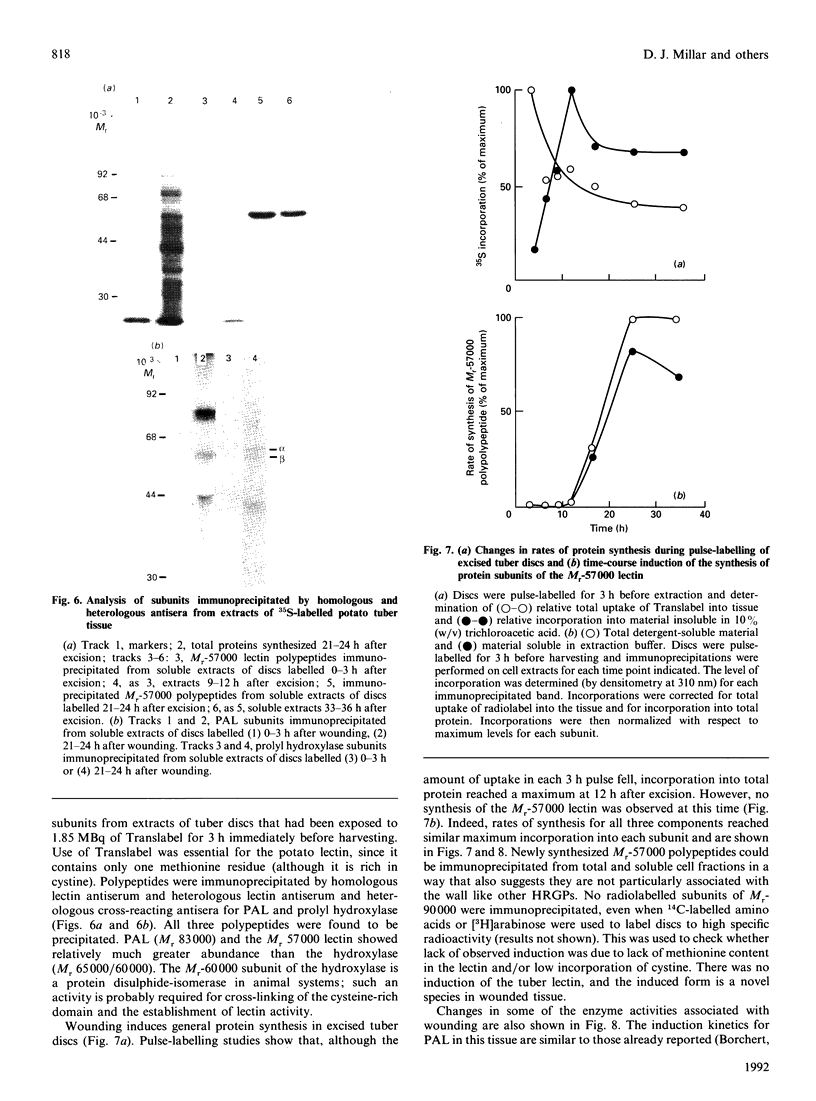
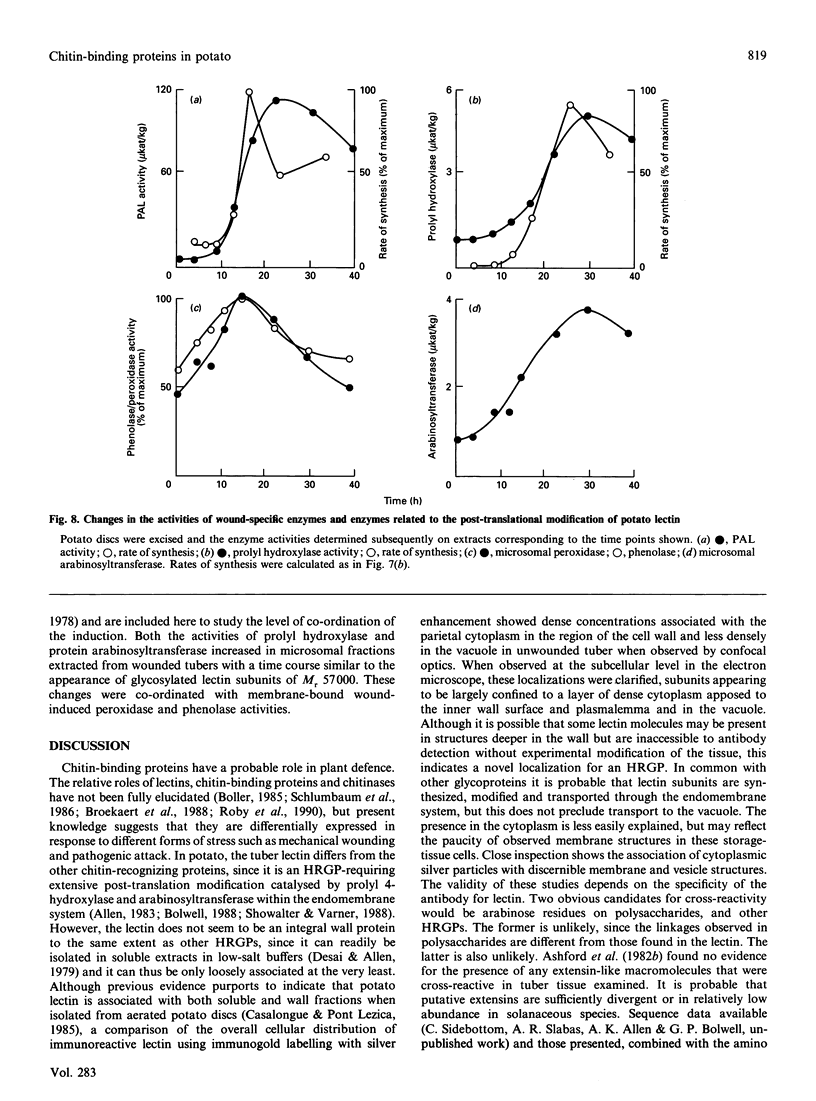
Tubers of potato (Solanum tuberosum L.) contain a number of chitin-binding proteins which have possible functions in defence against pathogens. A major protein of the tuber is the chitin-binding lectin which has been further characterized with respect to its antigenicity and N-terminal amino acid sequence. By using an antiserum monospecific for tuber lectin in unwounded potato the protein was found in the cytoplasm and vacuole, unusually for a hydroxyproline-rich glycoprotein, but consistent with its soluble nature in subcellular extracts. Little increased synthesis of the lectin precursor or the post-translationally modified form could be demonstrated in excised potato tuber discs. However, after wounding there is increased synthesis of another hydroxyproline-containing glycoprotein of Mr 57,000, which binds to chitin and shares common epitopes with the lectin. In comparison with the tuber lectin, this novel glycoprotein contains less hydroxyproline, but from its overall composition it is clearly not an underhydroxylated form of the tuber lectin. It differed in its N-terminal amino acid sequence and was much less glycosylated, although arabinose was still present. Synthesis of the Mr-57,000 polypeptide began after the initial burst of protein synthesis and increased, reaching a peak at 24 h after wounding. The protein was produced with its enzymes of post-translational modification, prolyl hydroxylase and arabinosyltransferase, concomitantly with the marker enzymes for wounding, phenylalanine ammonia-lyase and membrane-bound phenol oxidase and peroxidase.
Full text
PDF

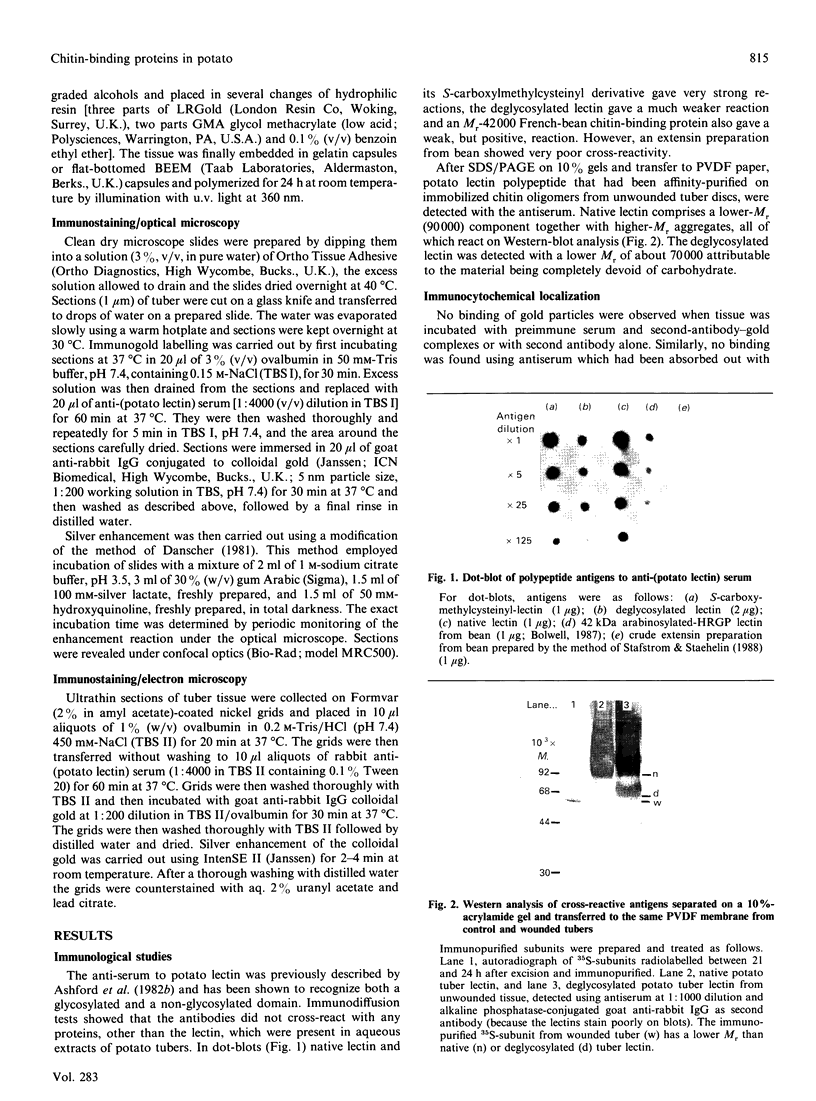
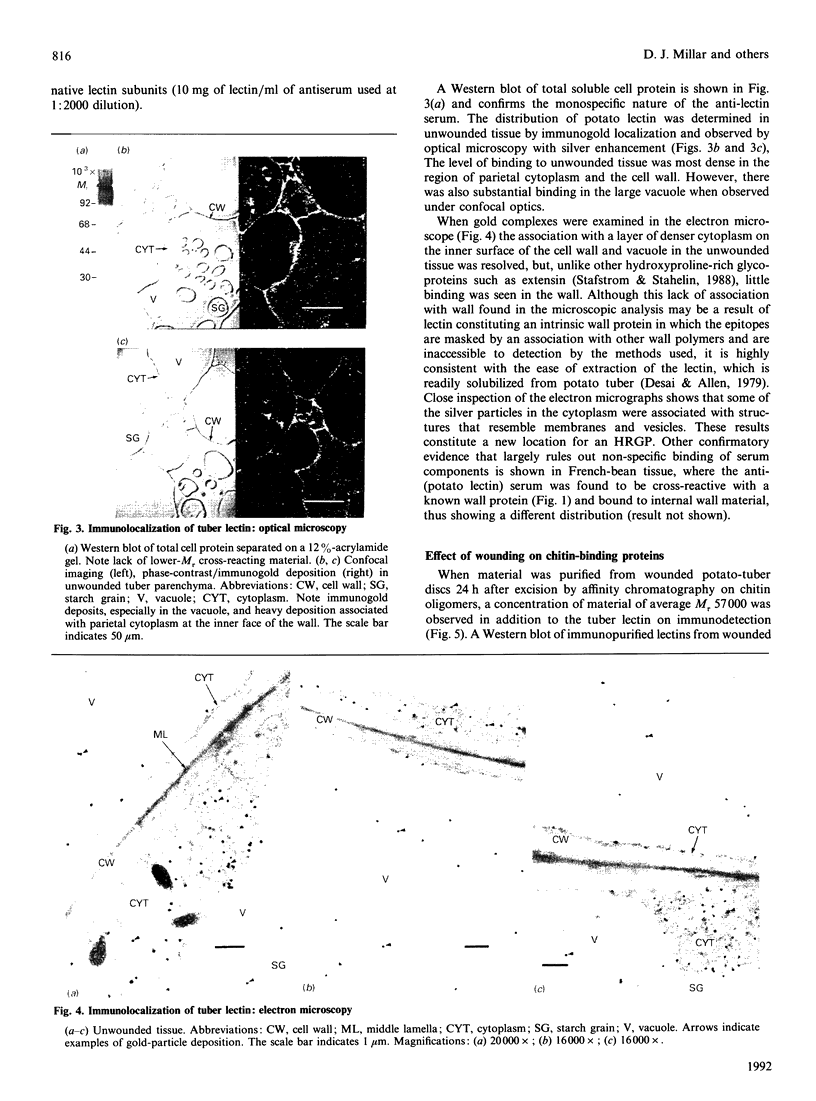
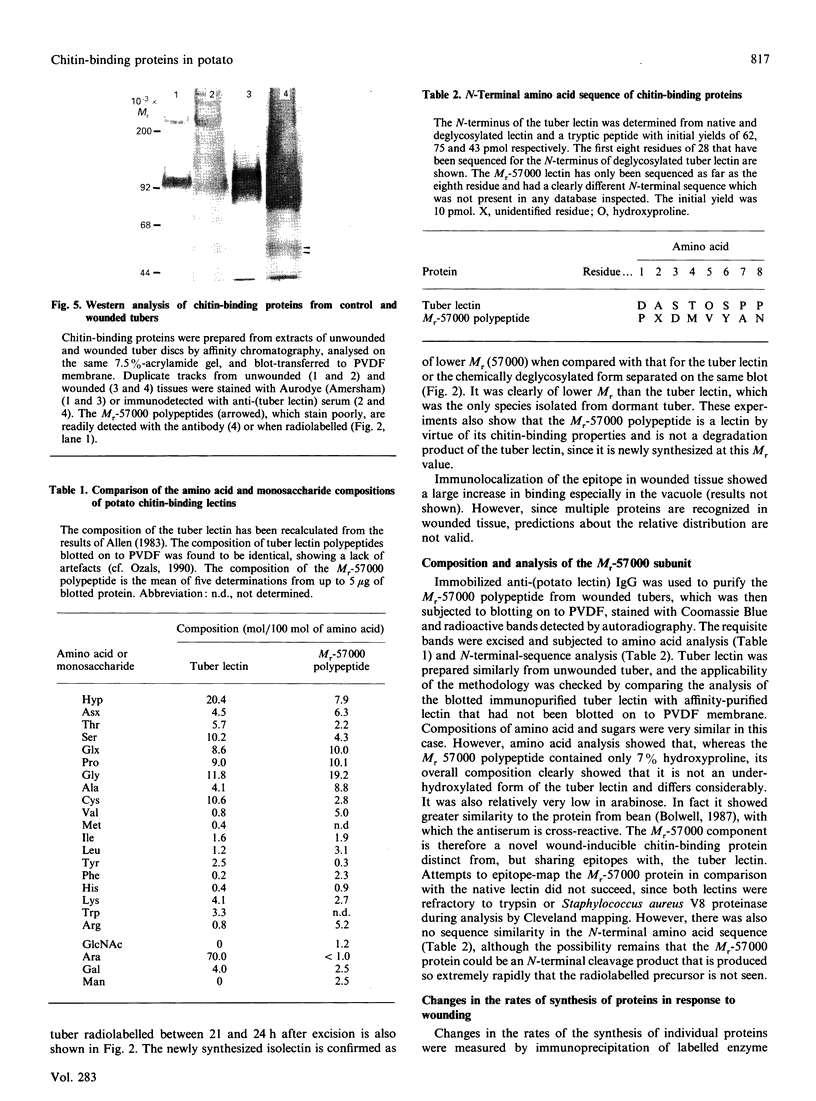


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Desai N. N., Neuberger A., Creeth J. M. Properties of potato lectin and the nature of its glycoprotein linkages. Biochem J. 1978 Jun 1;171(3):665–674. doi: 10.1042/bj1710665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Ellis J., Rivett D. E. The presence of glycoproteins in the cell membrane complex of a variety of keratin fibres. Biochim Biophys Acta. 1991 Jul 8;1074(2):331–333. doi: 10.1016/0304-4165(91)90172-d. [DOI] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A. The purification and properties of the lectin from potato tubers, a hydroxyproline-containing glycoprotein. Biochem J. 1973 Oct;135(2):307–314. doi: 10.1042/bj1350307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol I., Heinstein P. F., Low P. S. Rapid Stimulation of an Oxidative Burst during Elicitation of Cultured Plant Cells : Role in Defense and Signal Transduction. Plant Physiol. 1989 May;90(1):109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford D., Allen A. K., Neuberger A. The production and properties of an antiserum to potato (Solanum tuberosum) lectin. Biochem J. 1982 Mar 1;201(3):641–645. doi: 10.1042/bj2010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford D., Desai N. N., Allen A. K., Neuberger A., O'Neill M. A., Selvendran R. R. Structural studies of the carbohydrate moieties of lectins from potato (Solanum tuberosum) tubers and thorn-apple (Datura stramonium) seeds. Biochem J. 1982 Jan 1;201(1):199–208. doi: 10.1042/bj2010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Dixon R. A. Membrane-bound hydroxylases in elicitor-treated bean cells. Rapid induction of the synthesis of prolyl hydroxylase and a putative cytochrome P-450. Eur J Biochem. 1986 Aug 15;159(1):163–169. doi: 10.1111/j.1432-1033.1986.tb09847.x. [DOI] [PubMed] [Google Scholar]
- Bolwell G. P., Robbins M. P., Dixon R. A. Elicitor-induced prolyl hydroxylase from French bean (Phaseolus vulgaris). Localization, purification and properties. Biochem J. 1985 Aug 1;229(3):693–699. doi: 10.1042/bj2290693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert R. Time course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue. Plant Physiol. 1978 Nov;62(5):789–793. doi: 10.1104/pp.62.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W., Cook L., Vayda M. E. Hypoxic stress inhibits multiple aspects of the potato tuber wound response. Plant Physiol. 1990 May;93(1):264–270. doi: 10.1104/pp.93.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danscher G. Localization of gold in biological tissue. A photochemical method for light and electronmicroscopy. Histochemistry. 1981;71(1):81–88. doi: 10.1007/BF00592572. [DOI] [PubMed] [Google Scholar]
- Datta K., Schmidt A., Marcus A. Characterization of two soybean repetitive proline-rich proteins and a cognate cDNA from germinated axes. Plant Cell. 1989 Sep;1(9):945–952. doi: 10.1105/tpc.1.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N. N., Allen A. K., Neuberger A. The properties of potato (Solanum tuberosum) lectin after deglycosylation by trifluoromethanesulphonic acid. Biochem J. 1983 Apr 1;211(1):273–276. doi: 10.1042/bj2110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N. N., Allen A. K. The purification of potato lectin by affinity chromatography on an N,N',N''-triacetylchitotriose-Sepharose matrix. Anal Biochem. 1979 Feb;93(1):88–90. [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Espelie K. E., Franceschi V. R., Kolattukudy P. E. Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound-healing potato tuber tissue. Plant Physiol. 1986 Jun;81(2):487–492. doi: 10.1104/pp.81.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. C., Nagao R. T., Key J. L. Developmentally regulated expression of soybean proline-rich cell wall protein genes. Plant Cell. 1989 Sep;1(9):937–943. doi: 10.1105/tpc.1.9.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGendre N., Matsudaira P. Direct protein microsequencing from Immobilon-P Transfer Membrane. Biotechniques. 1988 Feb;6(2):154–159. [PubMed] [Google Scholar]
- Mauch F., Staehelin L. A. Functional Implications of the Subcellular Localization of Ethylene-Induced Chitinase and [beta]-1,3-Glucanase in Bean Leaves. Plant Cell. 1989 Apr;1(4):447–457. doi: 10.1105/tpc.1.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Roby D., Broglie K., Cressman R., Biddle P., Chet I. L., Broglie R. Activation of a Bean Chitinase Promoter in Transgenic Tobacco Plants by Phytopathogenic Fungi. Plant Cell. 1990 Oct;2(10):999–1007. doi: 10.1105/tpc.2.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumeau D., Maher E. A., Kelman A., Showalter A. M. Extensin and Phenylalanine Ammonia-Lyase Gene Expression Altered in Potato Tubers in Response to Wounding, Hypoxia, and Erwinia carotovora Infection. Plant Physiol. 1990 Jul;93(3):1134–1139. doi: 10.1104/pp.93.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N. M., Bolwell G. P., Smith C. Wound-induced phenylalanine ammonia-lyase in potato (Solanum tuberosum) tuber discs. Significance of glycosylation and immunolocalization of enzyme subunits. Biochem J. 1990 Apr 1;267(1):163–170. doi: 10.1042/bj2670163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford A., Bevan M., Northcote D. Differential expression within a family of novel wound-induced genes in potato. Mol Gen Genet. 1989 Jan;215(2):200–208. doi: 10.1007/BF00339718. [DOI] [PubMed] [Google Scholar]
- Strang A. M., Williams J. M., Ferguson M. A., Holder A. A., Allen A. K. Trypanosoma brucei brucei variant surface glycoprotein contains non-N-acetylated glucosamine. Biochem J. 1986 Mar 1;234(2):481–484. doi: 10.1042/bj2340481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner J. E., Lin L. S. Plant cell wall architecture. Cell. 1989 Jan 27;56(2):231–239. doi: 10.1016/0092-8674(89)90896-9. [DOI] [PubMed] [Google Scholar]