Abstract
Background:
Exercise training significantly improves cardiorespiratory fitness (CRF) in heart failure with reduced ejection fraction (HFrEF) patients, but high-intensity interval training (HIIT) is not superior to moderate-intensity interval training (MIIT). Whether HIIT is more beneficial than MIIT in patients with heart failure with preserved ejection fraction (HFpEF) remains unclear.
Methods:
On August 29, 2021, we conducted a comprehensive computerized literature search of the Medline, EMBASE, Web of Science, and Cochrane databases using the following keywords: “HF or diastolic HF or HFpEF or HF with normal ejection fraction and exercise training or aerobic exercise or isometric exercises or physical activity or cardiac rehabilitation.” Only randomized controlled trials (RCTs) reporting comparisons between HIIT and MIIT in HFpEF were included in the final analysis to maintain consistency and obtain robust pooled estimates. Methodological quality was assessed based on the ratings of individual biases. To generate an overall test statistic, the data were analyzed using the random-effects model for a generic inverse variance. Outcome measures were reported as an odds ratio, and confidence intervals (CIs) were set at 95%. The study followed PRISMA guidelines.
Results:
This meta-analysis included only RCTs comparing the efficacy of HIIT and MIIT in HFpEF patients. This study included 150 patients from 3 RCTs. In the current pooled data analysis, HIIT significantly improves diastolic function measured by E/A ratio (WMD, 0.13; 95% CI, 0.03–0.23, P = .009). However, no significant change was observed in the diastolic function measured by E/e’ ratio (WMD, 0.39; 95% CI, −2.40 to 3.18, P = .78), and CRF evaluated by both VO2 (mL/kg per min; WMD, −0.86; 95%CI, −5.27 to 3.55, P = .70) and VE/CO2 slope (WMD, 0.15; 95% CI, −10.24 to 10.53, P = .98), and systolic function (EF-WMD, −2.39; 95% CI, −12.16% to 7.38%, P = .63) between HIIT and MIIT in patients with HFpEF.
Conclusion:
In HFpEF patients, HIIT may be superior to MIIT in improving diastolic function, measured by E/A, but not CRF and left ventricular systolic function.
Keywords: heart function, HFpEF, high-intensity interval training, meta-analysis, moderate-intensity interval training
1. Introduction
Recent epidemic studies have reported that there are over 10 million heart failure patients worldwide, with more than half of them having heart failure with preserved ejection fraction (HFpEF).[1,2] Reduced exercise intolerance and dyspnea are the early symptoms of heart failure with reduced ejection fraction (HFrEF) and HFpEF patients.[3] Left ventricular diastolic dysfunction detected by echocardiography is essential for the diagnosis of HFpEF, and abnormal relaxation of the heart detected by echocardiography has been identified as one of the mechanisms of HFpEF.[4] In contrast to the high incidence of HFpEF, the lack of effective pharmacotherapy worsens the situation.[5,6] Consequently, an increasing number of researchers are focusing on nonpharmacotherapy interventions that could improve diastolic function and alleviate exercise intolerance in HFpEF.[7]
Cardiorespiratory fitness (CRF) is significantly increased in HFrEF patients who engage in interval-based exercise training at hospital or home.[8,9] Multiple studies found an association between interval exercise training and improved diastolic function[10] and exercise tolerance among HFpEF patients, particularly maximal exercise capacity measured by peak oxygen consumption in clinically stable patients with HFpEF.[11,12] In addition, high-intensity interval training (HIIT) may be superior to MIIT for improving exercise intolerance and diastolic function in HFpEF patients.[11,12] HIIT is a modality in which intervals of 1 to 4 minutes of greater intensity at a high submaximal load are interspersed with intervals of low to moderate-intensity.[13] Several small-scale randomized controlled trials (RCTs) on the efficacy of HIIT and MIIT in HFpEF patients have already been conducted. However, their results are debatable. This meta-analysis aims to evaluate the effects of HIIT and MIIT on CRF and heart function in HFpEF patients based on the previously conducted RCTs.
2. Methods
2.1. Data sources and searches
On August 29, 2021, we conducted a comprehensive computerized literature search of the Medline, EMBASE, Web of Science, and Cochrane databases. A research strategy that combined MeSH terms was implemented using the following keywords: “HF or diastolic HF or HFpEF or HF with normal EF and exercise training or aerobic exercise or isometric exercises or physical activity or cardiac rehabilitation.” In addition, we also searched the reference lists of selected articles and reviews (Supplemental Table 1, http://links.lww.com/MD/I494).
2.2. Study selection
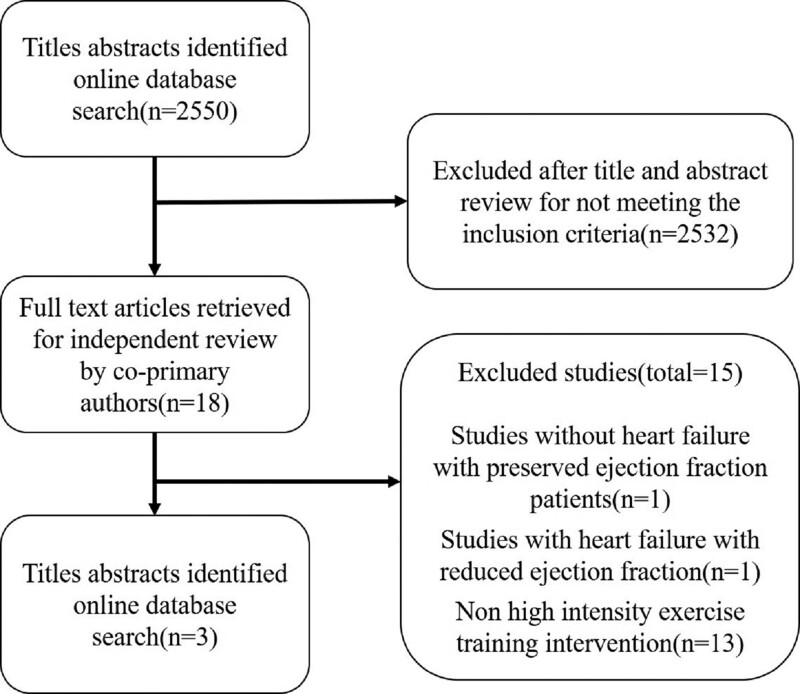
All comparative studies on exercise training in HFpEF, including randomized or nonrandomized parallel-group trials and observational studies, were included. Reviews, case reports, and conference proceedings that lacked primary data were excluded (Fig. 1). Only RCTs reporting comparing HIIT with MIIT in HFpEF were reported. At least one of the results, including changes in peak oxygen uptake in mL/kg per minute and VE/CO2 slope (stand for change in CRF) and markers of diastolic function such as E/A ratio, E/e’ ratio, left atrial volume index (LAVI), and early deceleration time (DT) and systolic function (measured by ejection fraction) were included in the final analysis to maintain consistency and obtain robust pooled estimates. The review protocol has been registered in PROSPERO: International Prospective Register of Systematic Reviews (CRD42021270782). Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021270782, Accessed August 29, 2021.
Figure 1.
Flow diagram for inclusion of studies in the meta-analysis.
2.3. Data extraction
Four authors researched and analyzed the original data (Jin-Hua Ye, Jin-hua Xue, Mu-jin Xie, and Ning Yang). The authors (Ping Lai and Jin-Hua Xue) downloaded full-text articles from PubMed if they believed they met the inclusion criteria after reading the titles and abstracts. A standardized questionnaire was used for data extraction, independently performed by the primary authors (Ping Lai, Mu-Jin Xie, and Jin-Hua Xue). We contacted the authors for original data if data was not provided in the publications. Any controversy regarding the study was resolved through discussion by the corresponding authors’ evaluation (Yi-Ming Zhong and Yong-Ling Liao).
2.4. Meta-analysis of performance and heterogeneity assessment
For each included study, all measurements relating to the indicators and their SD were independently imported into RevMan 5.3 software. The data were analyzed using the random-effects model for a generic inverse variance to produce an overall test statistic. Outcome measures were reported as an odds ratio, and confidence intervals (CIs) were set to 95%. The outcome was considered conclusive (or statistically significant, P < .05), if the 95% CI did not include 1.0. Forest plots were visually evaluated to determine “trends” in the data. Using the Higgins score (I2) the heterogeneity of the included studies was evaluated. Values of I2 were 0% to 40% might not be significant; 30% to 60% may indicate moderate heterogeneity; 50% to 90% may indicate substantial heterogeneity and 75% to 100% may indicate considerable heterogeneity.
3. Results
A total of 2550 articles were searched, and fifteen of the 18 full-text articles retrieved for all title abstracts were excluded because they did not meet the inclusion criteria (see Fig. 1 and Supplemental Table 2, http://links.lww.com/MD/I495).[14–28] Finally, 150 participants enrolled in 3 RCTs[11–13] published between 2014 and 2021, with a mean follow-up duration of 4 to 12 weeks (weighted mean duration, about 9 weeks) were included in this study. In all trials, cardiopulmonary exercise testing was adapted to assess exercise capability in all trials. All trials included patients with HFpEF who were well-compensated, stabilized on cardiac medications, and had not been hospitalized recently (the definition of HFpEF and exclusion criteria are given in Table 1). The baseline demographic and clinical characteristics of the study participants are summarized in Table 2. The exercise training protocol, control group protocol, and outcomes measured in the included trials are shown in Table 3. CRF assessment and echocardiographic at baseline and follow-up are shown in Tables 4 and 5.
Table 1.
HFPEF definition and exclusion criteria used in the studies included in the meta-analysis.
| Angadi et al[11] | Donelli et al[10] | Mueller et al[13] | |
|---|---|---|---|
| Criteria used for defining HFpEF | HFpEF diagnosis with NYHA heart failure Class II–III symptoms | All patients with signs and symptoms of heart failure, functional class II or III of the NYHA, LV ejection fraction > 50% and evidence of diastolic dysfunction with E/e’ above 15 were considered eligible. | Sedentary patients with signs and symptoms of HFpEF (exertional dyspnea [NYHA class II–III], LVEF of 50% or greater, and elevated estimated LV filling pressure [E/e′ medial ≥ 15] or E/e′ medial of 8 or greater with concurrent elevated natriuretic peptides [NT-pro BNP ≥ 220 pg/mL or BNP ≥ 80 pg/mL]) |
| Exclusion criteria | Subjects were excluded if they had unstable angina, myocardial infarction in the past 4 wks, uncompensated heart failure, NYHA class IV symptoms, complex ventricular arrhythmias (at rest or during the maximal exercise test), medical or orthopedic conditions that precluded treadmill walking, symptomatic severe aortic stenosis, acute pulmonary embolus, acute myocarditis, and medication noncompliance. | The presence of unstable ventricular arrhythmias, unstable or severe angina, moderate to severe valvular heart disease, anemia, and cognitive limitations in understanding the study protocol. Presence of pacemaker, autonomic neuropathy, and cardiomyopathy, moderate to severe pulmonary disease, recent acute cardiovascular event (<3 mo), congenital heart disease, symptomatic peripheral arterial disease or severe musculoskeletal diseases limiting exercise were also considered exclusion criteria. | nonHFpEF causes for HF symptom; inability to exercise, pulmonary disease (FEV1 < 50% predicted); Participation in another trial |
FEV1 = forced expiratory pressure in 1 sec, HF = heart failure, HFpEF = HF with preserved ejection fraction; NYHA = New York heart association, OR = odds ratio.
Table 2.
Baseline characteristics of the studies included in meta-analysis.
| Angadi et al[11] | Donelli et al[10] | Mueller et al[13] | |
|---|---|---|---|
| Total participants (control/training) | 6/9 | 9/10 | 58/58 |
| Women% | 20.0 | 12.0 | 65.5 |
| White% | NA | NA | NA |
| Mean age, yr | 70.0 ± 8.2 | 60.0 ± 9.5 | 70.0 ± 7.5 |
| Mean body mass index, kg/m2 | 29.6 ± 4.2 | 33.5 ± 5.5 | 30.6 ± 5.9 |
| NYHA Class II% | NA | 84.2 | 76 |
| NYHA Class III% | NA | 15.8 | 24 |
| Hypertension% | NA | 100 | 82.5 |
| Diabetes mellitus% | 27.0 | 57.9 | 26.7 |
| Base line systolic BP, mm Hg | 134 ± 18.0 | NA | 129 ± 13.5 |
| Baseline heart rate, Bpm | NA | NA | 65 ± 11 |
| Presentation EF, % | 65.4 ± 4.6 | 65.0 ± 5.0 | NA |
| Exercise capacity assessment | Cardiopulmonary exercise testing | Cardiopulmonary exercise testing | Cardiopulmonary exercise testing |
| 6-min walk at baseline, feet | NA | NA | NA |
| Peak oxygen uptake baseline, mL/kg per min | 18.3 ± 4.8 | 16.8 ± 3.4 | 18.6 ± 5.3 |
| Blinded assessment of outcome | NA | YES | YES |
Data represented as mean ± SD.
BP = blood pressure, EF = ejection fraction, NA = not available, NYHA = New York Heart Association.
Table 3.
Control and exercise group interventions used in the studies included in the meta-analysis.
| Angadi et al[11] | Donelli et al[10] | Mueller et al[13] | |
|---|---|---|---|
| Exercise training group intervention | Started with intervals of 2-min duration at 80%–85% PHR, separated by 2-min of recovery at 50% of PHR to achieve a total “on-time” of 16 min of high-intensity exercise. They were progressed by the start of the sec wk of training to completing 4, 4-min intervals at 85%–90% PHR, separated by 3-min at 50% PHR. | Treadmill; HIIT sessions consisted of a warm-up of 10 min at moderate-intensity, 4 intervals of 4-min at high-intensity, alternating with 3 intervals, and a 3-min cool down phase at moderate-intensity, totaling 38 min. High-intensity intervals were performed at 80%–90% of peak VO2 and 85%–95% of PHR, aiming at a RPE of 15–17. | High-intensity interval training was scheduled 3 times per wk for 38 min per session (10-min warm-up at 35%–50% of heart rate reserve, 4 × 4-min intervals at 80%–90% of heart rate reserve, interspaced by 3 min of active recovery) |
| Control group intervention | Began with 15 min of continuous exercise at 60% PHR, increasing to 30 min of continuous exercise at 70% PHR by the start of the 2nd wk. | Moderate-intensity was considered 50%–60% of peak VO2 and 60%–70% of peak HR, corresponding to 11–13 on the Borg RPE scale. MIT group trained for 47 min at moderate-intensity to match the total relative work of both protocols. | MIT continuous training was scheduled 5 times per week for 40 min per session (35%–50% of heart rate reserve). |
| Duration | 4 wks | 12 wks | 12 wks |
| Outcome measured | Cardiopulmonary (peak VO2) exercise test; Assessment of left ventricular function by echocardiography, and Endothelium-dependent dilation of the brachial artery was measured by B-mode ultrasound (Terason t3000, Burlington, MA) using Brachial Artery Reactivity Task Force guidelines | Physical examination, Doppler echocardiography, CPET and blood sampling and responded to a QoL questionnaire. | Medical history, physical examination, anthropometry, electrocardiogram, blood analysis, cardiopulmonary exercise testing, echocardiography, and the Kansas City Cardiomyopathy questionnaire. |
HIIT = high-intensity interval training, LV = left ventricle, Peak VO2 = peak oxygen uptake, PHR = peak heart rate.
Table 4.
Baseline and follow-up parameters of echocardiography.
| Angadi et al[11] | Donelli et al [10] | Mueller et al[13] | ||
|---|---|---|---|---|
| E/A baseline (mL/kg/min) | HIT | 1.3 ± 0.5 | 0.99 ± 0.2 | NA |
| MIT | 1.3 ± 0.5 | 0.99 ± 0.2 | NA | |
| E/A follow-up (mL/kg/min) | HIT | 1.2 ± 0.5 | 0.91 ± 0.2 | NA |
| MIT | 1.6 ± 1.1 | 1.08 ± 0.3 | NA | |
| E/e’ baseline | HIT | 14.6 ± 5.6 | 14.24 ± 2.0 | 15.8 ± 3.7 |
| MIT | 17.7 ± 6.3 | 13.3 ± 3.0 | 15.9 ± 4.1 | |
| E/e’ follow-up | HIT | 12.7 ± 4.7 | 11.6 ± 3.0 | 15.2 ± 4.8 |
| MIT | 16.7 ± 5.2 | 11.1 ± 2.0 | 15.6 ± 5.0 | |
| LAVI baseline (mL) | HIT | 35.8 ± 3.0 | 47.0 ± 10.0 | 35.4 ± 9.0 |
| MIT | 40.5 ± 9.3 | 42.0 ± 8.0 | 37.9 ± 13.0 | |
| LAVI follow-up (mL) | HIT | 32.4 ± 7.2 | 46.0 ± 12.0 | 35.2 ± 10.2 |
| MIT | 46.3 ± 18.1 | 42.0 ± 9.0 | 36.8 ± 10.5 | |
| DT baseline (ms) | HIT | 194.0 ± 55.0 | 233.0 ± 33.0 | NA |
| MIT | 199.0 ± 71.0 | 214.0 ± 33.0 | NA | |
| DT follow-up (ms) | HIT | 225.0 ± 40.0 | 222.0 ± 27.0 | NA |
| MIT | 220.0 ± 43.0 | 209.0 ± 37.0 | NA | |
| EF baseline (%) | HIT | 65.0 ± 5.0 | 65.0 ± 5.0 | NA |
| MIT | 66.0 ± 4.0 | 65.0 ± 5.0 | NA | |
| EF follow-up (%) | HIT | 63.0 ± 6.0 | 66.0 ± 4.0 | NA |
| MIT | 61.0 ± 5.0 | 65.0 ± 5.0 | NA | |
EF = ejection fraction, HIIT = high-intensity interval training, LAVI = left atrial volume index, MIIT = moderate-intensity interval training, NA = not available.
Table 5.
Baseline and follow-up parameters of CRF.
| Angadi et al[11] | Donelli et al[10] | Mueller et al[13] | ||
|---|---|---|---|---|
| Peak oxygen uptake baseline (mL/kg/min) | HIT | 19.2 ± 5.2 | 16.1 ± 3.3 | 18.9 ± 5.4 |
| MIT | 16.9 ± 3.0 | 17.6 ± 3.5 | 18.2 ± 5.1 | |
| Peak oxygen uptake follow-up (mL/kg/min) | HIT | 21.0 ± 5.2 | 19.6 ± 3.5 | 20.2 ± 6.0 |
| MIT | 16.8 ± 3.0 | 19.5 ± 3.7 | 19.8 ± 5.8 | |
| VE/VCO2 slope baseline | HIT | 31.2 ± 11.5 | 39.4 ± 6.1 | 34.5 ± 7.9 |
| MIT | 26.4 ± 2.4 | 36.8 ± 5.4 | 34.2 ± 7.2 | |
| VE/VCO2 slope follow-up | HIT | 31.6 ± 10.3 | 35.7 ± 4.7 | 35.0 ± 9.8 |
| MIT | 26.7 ± 3.1 | 34.6 ± 5.1 | 33.7 ± 6.8 | |
CRF = cardiorespiratory fitness, HIT = high-intensity interval, MIT = moderate-intensity interval.
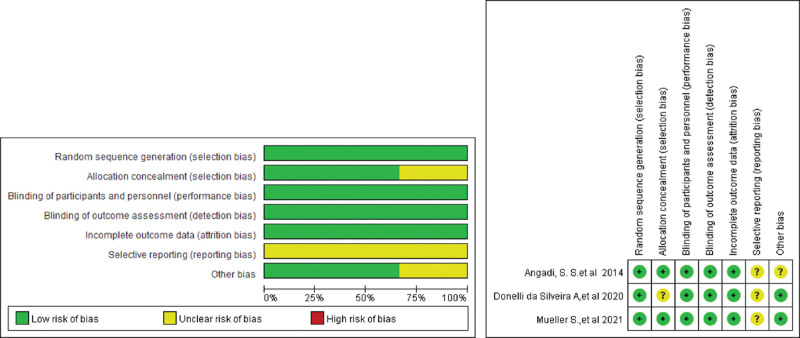
3.1. Quality assessment
The quality assessment for this study was performed by the Cochrane risk of bias assessment tool (shown in Fig. 2). All studies exhibited random sequence generation, incomplete outcome data, and blinded assessment outcomes in the results. Selective reporting of results was not observed in any of the selected studies.
Figure 2.
Risk of bias assessment in the included clinical trials.
3.2. Diastolic and systolic function
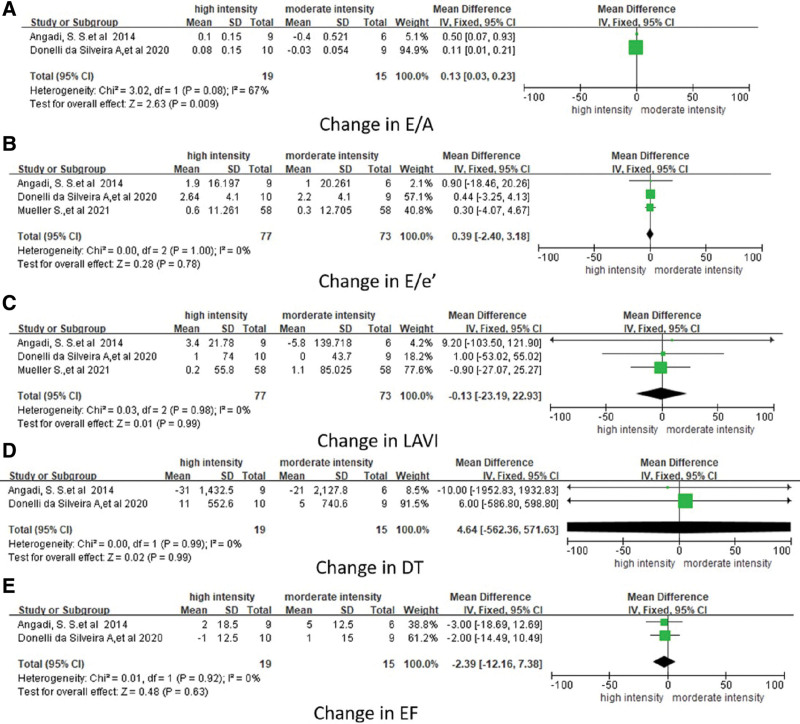
All studies reported the effect on the diastolic function of HIIT and MIIT. In 3 studies, E/e’ and LAVI were reported as diastolic function parameter, whereas DT and E/A were only reported in 2 trials (Table 4). Pooling across all the available studies using fixed-effect meta-analysis showed a significant change in E/A (WMD, 0.13; 95% CI, 0.03–0.23; P = .009; Fig. 3a), but no significant changes in E/e’ (WMD, 0.39; 95% CI, −2.40 to 3.18; P = .78; Fig. 3b), LAVI (WMD, 0.13; 95% CI, −23.19 to 22.93; P = .99; Fig. 3c), and DT (ms; WMD, 4.64; 95% CI, −562.36 to 571.63; P = .99; Fig. 3d) with HIIT when compared with the MIIT participants. Two studies used ejection fraction (EF) to evaluate systolic function (Table 4). Pooling across all the available studies using fixed-effect meta-analysis showed no significant difference in systolic function between HIIT and MIIT evaluated by EF (WMD, −2.39; 95% CI, −12.16% to 7.38%; P = .63; Fig. 3e).
Figure 3.
Forest plot showing the effect of HIIT and MIIT on left ventricular diastolic function (A: change in E/A, B: change in E/e’, C: change in LAVI, D: change in DT,; and E: systolic function (change in EF) among participants included. CI = confidence interval, HIIT = high-intensity interval training, LAVI = left atrial volume index, MIIT = moderate-intensity interval training, WMD = weighted mean difference.
3.3. Cardiorespiratory fitness
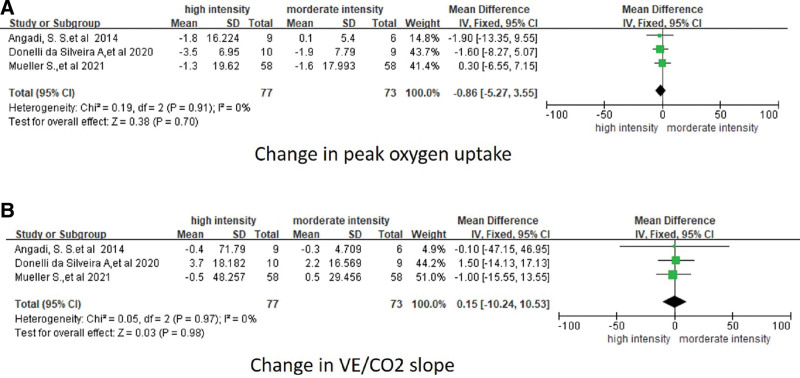
All trials recorded exercise capacity at baseline and after exercise training elevated by symptom-limited cardiopulmonary exercise testing on a bicycle ergometer or treadmill. In 2 studies, patients with HFpEF who underwent HIIT had a greater increase in peak oxygen uptake than those who underwent MIIT. In contrast, in the third study, there was no significant difference between the 2 types of exercise (Table 5). Fixed-effect meta-analysis of these 3 trials showed that HIIT exercise training appears to be associated with an increase in peak oxygen uptake (ml/kg per min) from baseline to follow-up among patients with HFpEF, although this association was not statistically significant (WMD, −0.86; 95% CI, −5.27 to 3.55; P = .7; see Table 6 and Fig. 4a). The VE/VCO2 slope is also an important index to evaluate the cardiopulmonary function, and 3 trials adapted to this index during their study. One of them showed the VE/VCO2 slope decreased after both HIIT and MIIT, but the fixed-effect meta-analysis of those trials showed that HIIT exercise training did not significantly reduce the VE/VCO2 slope (WMD, 0.15; 95% CI, −10.24 to 10.53, P = .98) compared with MIIT (Fig. 4b). In addition, there was no statistically significant heterogeneity between studies reporting peak oxygen uptake (I = 0.19).
Table 6.
Exercise-associated adverse events as reported in the studies included in the meta-analysis.
| Angadi et al[11] | Donelli et al[10] | Mueller et al[13] | |
|---|---|---|---|
| Exercise-associated adverse events | NA | No serious adverse event, back pain (n = 1), knee pain (n = 1), epilepsy (n = 1) | No serious adverse event, Two adverse events in MIIT group |
MIIT = moderate-intensity interval training, NA = not available.
Figure 4.
Forest plot showing the effect of HIIT and MIIT on cardiorespiratory fitness. (A) Measured as peak oxygen uptake (mL/kg per min) and (B) measured as VE/CO2 slope, among included studies. CI = confidence interval, HIIT = high-intensity interval training, WMD = weighted mean difference.
3.4. Safety of exercise training
In the included studies, no major adverse effects of exercise training were reported (Table 6).
4. Discussion
This is the first meta-analysis that compares the effects of HIIT and MIIT on HFpEF. Although only 3 studies were included in the present meta-analysis, the fact that the quality of the evidence for the analyzed outcome was determined to be moderate to strong demonstrates the reliability of this meta-analysis. In addition, we discovered something intriguing. First, HIIT does not outperform MIIT in improving CRF in patients with HFpEF. Second, while HIIT appears to improve E/A, there is no significant improvement in E/e’, DT, LAVI, or systolic function (EF). Collectively, these findings indicate that HIIT has no advantage in terms of improving CRF or systolic function. Consider the diastolic function, which may be associated with the evaluating index.
Exercise intolerance is a common symptom of both HFpEF and HErEF. The precise mechanism of HFpEF is still unknown, and there is no effective treatment for halting or reversing its progression. Exercise training has been shown to improve exercise tolerance and heart function in patients with HFrEF or HFpEF.[29] However, different intensities of exercise have different roles in improving HFrEF, HIIT was not superior to MIIT in preventing left ventricular remodeling or aerobic capacity[9] A meta-analysis showed that HIIT improves peak VO2 and should be considered as a component of care for HFrEF patients.[8] Studies on HIIT and MIIT in HFpEF remain controversial.
Despite a few small studies concluding that HIIT improved the exercise capacity in HFpEF, further research is required. A current meta-analysis concluded that HIIT could not improve peak VO2 and VE/VCO2 slopes. In addition, we had to acknowledge that exercise duration varies between studies and that these differences in duration may result in different outcomes. Few studies used the VE/VCO2 slope as an evaluating parameter in HFpEF.[11–13] Although the VE/VCO2 slope is important for predicting long-term outcomes in HFpEF,[30] a steep VE/VCO2 slope is predictive of an increased risk of cardiovascular events.[31] The VE/VCO2 slope should be included and analyzed in further studies.
Diastolic dysfunction is a distinguishing feature of HFpEF patients; E/A, E/e’, DT, and LAVI are important parameters for assessing the heart diastolic function.[4,32] Echocardiography is a noninvasive and low-cost diagnostic method. In echocardiography, HFpEF is characterized by an E-wave less than an A wave in wave doppler and a decrease in ventricle wall movement.[4,33] DT refers to the time interval between the peak of the E-wave and its projected baseline; it is also an important index for evaluating the diastolic function of the heart. In addition, LAVI refers to the left atrial volume index rather than left atrial pressure, which decreased with the progress of HFpEF.[34] In 2 of the 3 studies included in this meta-analysis, E/A and DT were used as indexes to evaluate the diastolic function, whereas E/e’ and LAVI were used to evaluate the diastolic function in all 3 studies.[11–13] Two studies concluded that HIIT did not improve diastolic function regardless of the index used to assess diastolic function. However, the pooled analysis shows that HIIT improves the E/A ratio in the HFpEF but has no effect on other index assess diastolic function indices. E/e’ parameter analysis by tissue doppler is a more reliable way to assess the diastolic function of the heart, making it difficult to determine whether HIIT is superior to MIIT in improving diastolic function.[35] E/A, DT, and LAVI would be modified based on various stages of HFpEF.[36]
The current study has a few limitations. First, due to the difficulty for physicians and patients, there is low compliance with exercise treatment during the exercise operation. Most studies on exercise interval training have a small number of participants, which is a problem in this meta-analysis, despite our efforts to find more studies without time constraints. HFpEF has drawn the attention of physicians over the past 2 decades, and more RCTs are being conducted, so larger-scale RCTs should confirm our results. Second, the small sample size of the RCTs included in this meta-analysis is a significant limitation, despite our efforts to reduce any inherent biases. Thirdly, only 1 RCT adapted life quality evaluation in HFpEF, although the importance of life quality evaluation is determining the efficacy of exercise on HFrEF and HFpEF.
In conclusion, HIIT is not superior to MIIT in HFpEF, and larger-scale studies are required to determine whether HIIT is more beneficial for HFpEF. To improve our understanding of the effects of exercise on HFpEF patients, we must also determine the optimal exercise intensity to identify the outcomes.
Author contributions
Conceptualization: Yong-Ling Liao, Yi-Ming Zhong.
Data curation: Mu-Jin Xie, Ning Yang.
Formal analysis: Mu-Jin Xie, Jin-Hua Ye, Yi-Ming Zhong.
Funding acquisition: Ping Lai, Yi-Ming Zhong.
Investigation: Yong-Ling Liao, Ping Lai, Jin-Hua Xue.
Methodology: Yong-Ling Liao, Ping Lai, Jin-Hua Xue, Mu-Jin Xie, Jin-Hua Ye, Ning Yang.
Supervision: Yong-Ling Liao.
Writing – original draft: Ping Lai.
Writing – review & editing: Yong-Ling Liao, Jin-Hua Xue, Yi-Ming Zhong.
Supplementary Material
PL, J-HX, and M-JX contributed equally to this work.
Abbreviation:
- CI
- confidence interval
- CRF
- cardiorespiratory fitness, HFrEF = heart failure with reduced ejection fraction
- DT
- deceleration time
- HFpEF
- heart failure with preserved ejection fraction
- HIIT
- high-intensity interval training
- LAVI
- left atrial volume index
- MIIT
- moderate-intensity interval training
- RCTs
- randomized controlled trials
Supplemental Digital Content is available for this article.
As no human beings or experimental subjects were included in the study, ethical approval is not necessary.
The authors have no conflicts of interest to disclose.
This work was supported by Natural Science Foundation of Jiangxi Province (No. 20202BABL206012), the Open Project of Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education (No. XN201804), Science and Technology Research Project of Education Department of Jiangxi Province (No. 170865), and Gannan Medical University Project (No. YB201803).
All data generated or analyzed during this study are included in this published article [and its supplementary information files];The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Lai P, Xue J-H, Xie M-J, Ye J-H, Yang N, Zhong Y-M, Liao Y-L. High-intensity and moderate-intensity interval training in heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Medicine 2023;102:8(e33010).
Contributor Information
Ping Lai, Email: laiping0202@163.com.
Jin-Hua Xue, Email: yejinhua@gmu.edu.cn.
Mu-Jin Xie, Email: xiemujin@163.com.
Jin-Hua Ye, Email: yejinhua@gmu.edu.cn.
Ning Yang, Email: yangning@163.com.
Yi-Ming Zhong, Email: yimingzhong@gmu.edu.cn.
References
- [1].Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–73. [DOI] [PubMed] [Google Scholar]
- [2].Shah SJ, Borlaug BA, Kitzman DW, et al. Research priorities for heart failure with preserved ejection fraction: national heart, lung, and blood institute working group summary. Circulation. 2020;141:1001–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed). 2016;69:1167. [DOI] [PubMed] [Google Scholar]
- [4].Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imag. 2020;13(1 Pt 2):245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zouein FA, de Castro Bras LE, da Costa DV, et al. Heart failure with preserved ejection fraction: emerging drug strategies. J Cardiovasc Pharmacol. 2013;62:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- [7].Lam CSP, Voors AA, de Boer RA, et al. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J. 2018;39:2780–92. [DOI] [PubMed] [Google Scholar]
- [8].Gomes Neto M, Duraes AR, Conceicao LSR, et al. High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: a systematic review and meta-analysis. Int J Cardiol. 2018;261:134–41. [DOI] [PubMed] [Google Scholar]
- [9].Ellingsen O, Halle M, Conraads V, et al. High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation. 2017;135:839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barr LA, Lambert JP, Shimizu Y, et al. Exercise training provides cardioprotection by activating and coupling endothelial nitric oxide synthase via a beta3-adrenergic receptor-AMP-activated protein kinase signaling pathway. Med Gas Res. 2017;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Donelli da SA, Beust de Lima J, da Silva Piardi D, et al. High-intensity interval training is effective and superior to moderate continuous training in patients with heart failure with preserved ejection fraction: a randomized clinical trial. Eur J Prev Cardiol. 2020;27:1733–43. [DOI] [PubMed] [Google Scholar]
- [12].Angadi SS, Mookadam F, Lee CD, et al. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol (1985). 2015;119:753–8. [DOI] [PubMed] [Google Scholar]
- [13].Mueller S, Winzer EB, Duvinage A, et al. Effect of high-intensity interval training, moderate continuous training, or guideline-based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2021;325:542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gary RA, Sueta CA, Dougherty M, et al. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004;33:210–8. [DOI] [PubMed] [Google Scholar]
- [15].Gary R. Exercise self-efficacy in older women with diastolic heart failure: results of a walking program and education intervention. J Gerontol Nurs. 2006;32:31–9. quiz4031. [DOI] [PubMed] [Google Scholar]
- [16].Karapolat H, Demir E, Bozkaya YT, et al. Comparison of hospital-based versus home-based exercise training in patients with heart failure: effects on functional capacity, quality of life, psychological symptoms, and hemodynamic parameters. Clin Res Cardiol. 2009;98:635–42. [DOI] [PubMed] [Google Scholar]
- [17].Kitzman DW, Brubaker PH, Morgan TM, et al. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Edelmann F, Gelbrich G, Düngen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–91. [DOI] [PubMed] [Google Scholar]
- [19].Alves AJ, Ribeiro F, Goldhammer E, et al. Exercise training improves diastolic function in heart failure patients. Med Sci Sports Exerc. 2012;44:776–85. [DOI] [PubMed] [Google Scholar]
- [20].Murad K, Brubaker PH, Fitzgerald DM, et al. Exercise training improves heart rate variability in older patients with heart failure: a randomized, controlled, single-blinded trial. Congest Heart Fail. 2012;18:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Smart NA, Haluska B, Jeffriess L, et al. Exercise training in heart failure with preserved systolic function: a randomized controlled trial of the effects on cardiac function and functional capacity. Congest Heart Fail. 2012;18:295–301. [DOI] [PubMed] [Google Scholar]
- [22].Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rustad LA, Nytrøen K, Amundsen BH, et al. One year of high-intensity interval training improves exercise capacity, but not left ventricular function in stable heart transplant recipients: a randomised controlled trial. Eur J Prev Cardiol. 2014;21:181–91. [DOI] [PubMed] [Google Scholar]
- [24].Suchy C, Massen L, Rognmo O, et al. Optimising exercise training in prevention and treatment of diastolic heart failure (OptimEx-CLIN): rationale and design of a prospective, randomised, controlled trial. Eur J Prev Cardiol. 2014;21(2 Suppl):18–25. [DOI] [PubMed] [Google Scholar]
- [25].Nolte K, Herrmann-Lingen C, Wachter R, et al. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex-DHF-P trial. Eur J Prev Cardiol. 2015;22:582–93. [DOI] [PubMed] [Google Scholar]
- [26].Pandey A, Kitzman DW, Brubaker P, et al. Response to endurance exercise training in older adults with heart failure with preserved or reduced ejection fraction. J Am Geriatr Soc. 2017;65:1698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Edelmann F, Anna Bobenko A, Gelbrich G, et al. Exercise training in diastolic heart failure (Ex-DHF): rationale and design of a multicentre, prospective, randomized, controlled, parallel group trial. Eur Heart J. 2017;19:501–2. [DOI] [PubMed] [Google Scholar]
- [28].Brubaker PH, Avis T, Rejeski WJ, et al. Exercise training effects on the relationship of physical function and health-related quality of life among older heart failure patients with preserved ejection fraction. J Cardiopulm Rehabil Prev. 2020;40:427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cattadori G, Segurini C, Picozzi A, et al. Exercise and heart failure: an update. ESC Heart Fail. 2018;5:222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nadruz W, Jr., West E, Sengelov M, et al. Prognostic value of cardiopulmonary exercise testing in heart failure with reduced, midrange, and preserved ejection fraction. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chase PJ, Kenjale A, Cahalin LP, et al. Effects of respiratory exchange ratio on the prognostic value of peak oxygen consumption and ventilatory efficiency in patients with systolic heart failure. JACC Heart Fail. 2013;1:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pieske B, Tschope C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the heart failure association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–317. [DOI] [PubMed] [Google Scholar]
- [33].Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868–77. [DOI] [PubMed] [Google Scholar]
- [34].Sharma AK, Kumar H, Razi MM, et al. To determine the correlation between echocardiographic diastolic parameters and invasively measured left ventricular end diastolic pressure in patients with heart failure with preserved ejection fraction—an observational, descriptive study. (CEAL-HFpEF study). Indian Heart J. 2021;73:470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen ZW, Huang CY, Cheng JF, et al. Stress echocardiography-derived E/e’ predicts abnormal exercise hemodynamics in heart failure with preserved ejection fraction. Front Physiol. 2019;10:1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shuai XX, Chen YY, Lu YX, et al. Diagnosis of heart failure with preserved ejection fraction: which parameters and diagnostic strategies are more valuable? Eur J Heart Fail. 2011;13:737–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.