Abstract
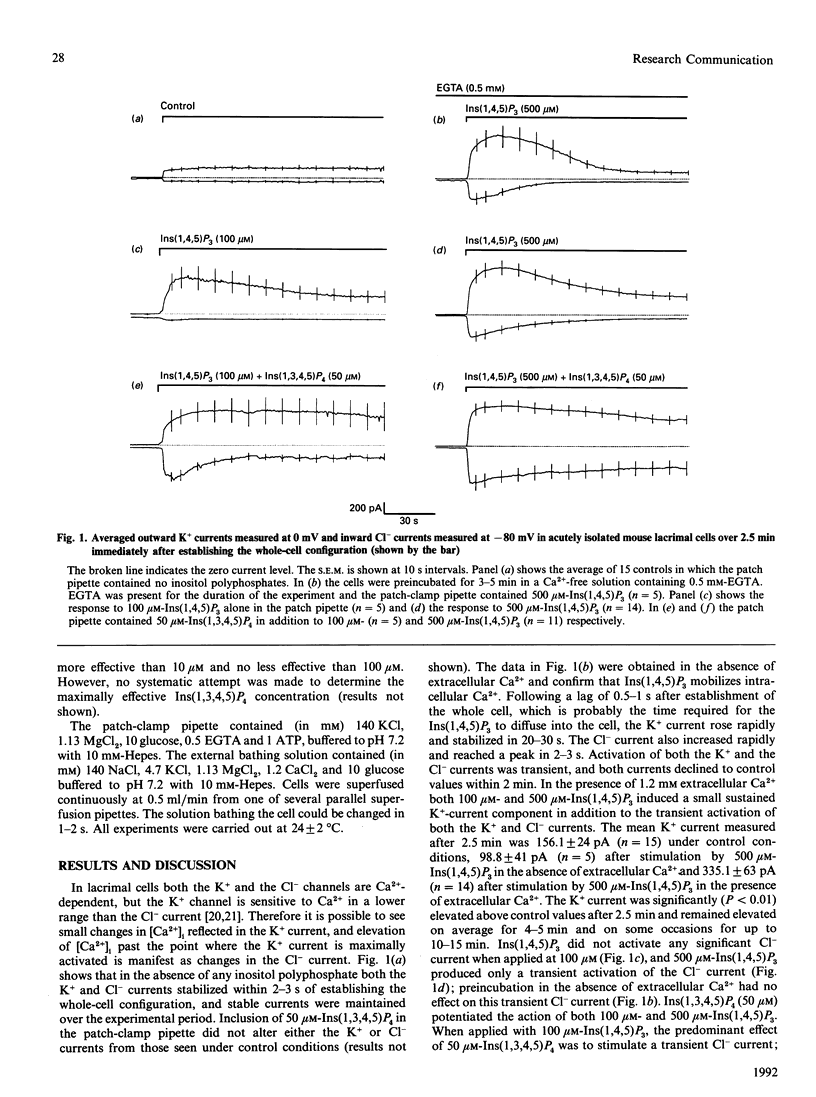
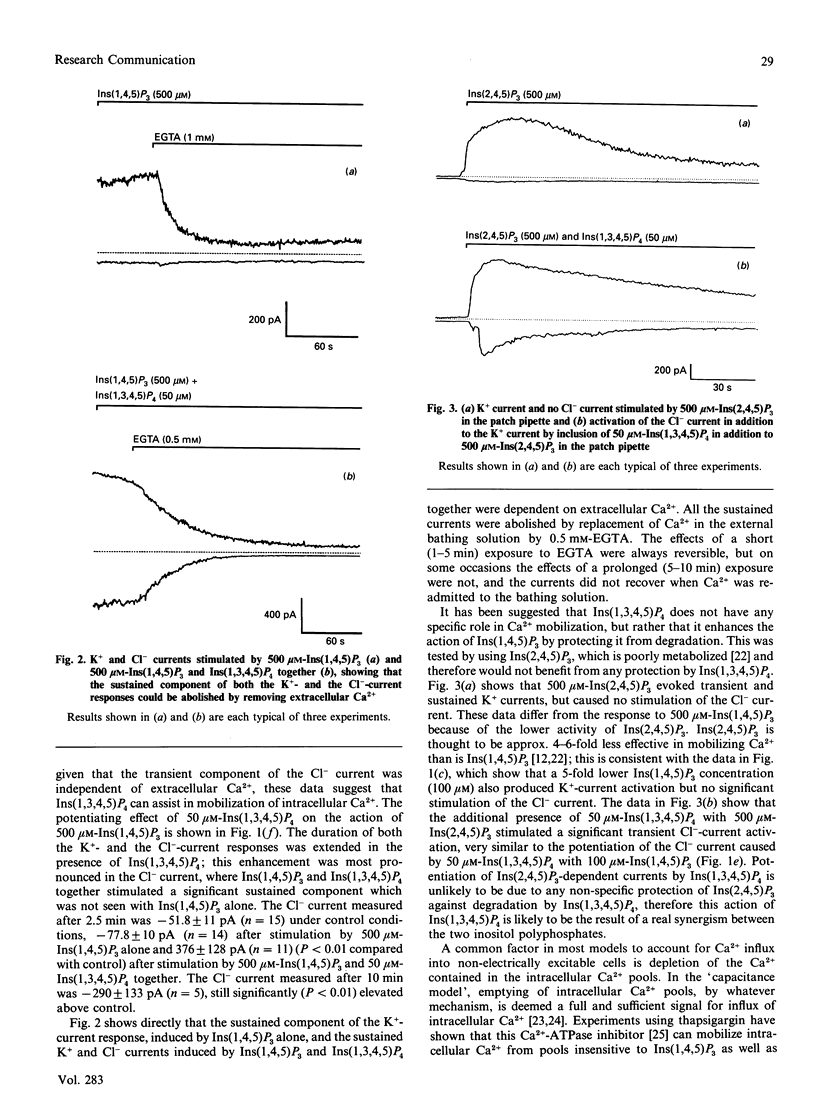
Infusion of 50 microM-Ins(1,3,4,5)P4 in addition to 500 microM-Ins(1,4,5)P3 into mouse lacrimal cells via a patch-clamp pipette promoted sustained activation of the Ca(2+)-dependent Cl- current, which could not be achieved with 500 microM-Ins(1,4,5)P3 alone. It has been proposed that Ins(1,3,4,5)P4 facilitates Ca2+ influx in the presence of Ins(1,4,5)P3 [Morris, Gallacher, Irvine & Petersen (1987) Nature (London) 330, 653-655], but a subsequent study in mouse lacrimal cells [Bird, Rossier, Hughes, Shears, Armstrong & Putney (1991) Nature (London) 352, 162-165] showed that a high concentration of Ins(1,4,5)P3 could mobilize both intra- and extra-cellular Ca2+ in the absence of Ins(1,3,4,5)P4. My data confirm these findings, but also show that Ins(1,3,4,5)P4 can stimulate additional Ca2+ influx even when the Ins(1,4,5)P3-dependent intracellular Ca2+ pools have been depleted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Willcocks A. L., Mulloy B., Potter B. V., Nahorski S. R. Characterization of inositol 1,4,5-trisphosphate- and inositol 1,3,4,5-tetrakisphosphate-binding sites in rat cerebellum. Biochem J. 1991 Mar 15;274(Pt 3):861–867. doi: 10.1042/bj2740861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Potter B. V., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 1989 Jul;109(1):85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Irvine R. F., Dawson A. P. Synergistic control of Ca2+ mobilization in permeabilized mouse L1210 lymphoma cells by inositol 2,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate. Biochem J. 1990 Oct 15;271(2):549–553. doi: 10.1042/bj2710549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donié F., Hülser E., Reiser G. High-affinity inositol 1,3,4,5-tetrakisphosphate receptor from cerebellum: solubilization, partial purification and characterization. FEBS Lett. 1990 Jul 30;268(1):194–198. doi: 10.1016/0014-5793(90)81006-a. [DOI] [PubMed] [Google Scholar]
- Downes C. P. Twenty-fifth Colworth medal lecture. The cellular functions of myo-inositol. Biochem Soc Trans. 1989 Apr;17(2):259–268. doi: 10.1042/bst0170259. [DOI] [PubMed] [Google Scholar]
- Ely J. A., Hunyady L., Baukal A. J., Catt K. J. Inositol 1,3,4,5-tetrakisphosphate stimulates calcium release from bovine adrenal microsomes by a mechanism independent of the inositol 1,4,5-trisphosphate receptor. Biochem J. 1990 Jun 1;268(2):333–338. doi: 10.1042/bj2680333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. E., Han J. K., Kao J. P., Nuccitelli R. The effects of inositol trisphosphates and inositol tetrakisphosphate on Ca2+ release and Cl- current pattern in the Xenopus laevis oocyte. Exp Cell Res. 1991 Feb;192(2):352–365. doi: 10.1016/0014-4827(91)90052-v. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Findlay I., Petersen O. H. Acetylcholine stimulates a Ca2+-dependent C1- conductance in mouse lacrimal acinar cells. Pflugers Arch. 1985 Mar;403(3):328–330. doi: 10.1007/BF00583609. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivorra I., Gigg R., Irvine R. F., Parker I. Inositol 1,3,4,6-tetrakisphosphate mobilizes calcium in Xenopus oocytes with high potency. Biochem J. 1991 Jan 15;273(Pt 2):317–321. doi: 10.1042/bj2730317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Second messenger-activated calcium influx in rat peritoneal mast cells. J Physiol. 1989 Nov;418:105–130. doi: 10.1113/jphysiol.1989.sp017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- Nunn D. L., Taylor C. W. Liver inositol, 1,4,5-trisphosphate-binding sites are the Ca2(+)-mobilizing receptors. Biochem J. 1990 Aug 15;270(1):227–232. doi: 10.1042/bj2700227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Gallacher D. V. Extracellular ATP activates receptor-operated cation channels in mouse lacrimal acinar cells to promote calcium influx in the absence of phosphoinositide metabolism. FEBS Lett. 1990 May 7;264(1):130–134. doi: 10.1016/0014-5793(90)80782-e. [DOI] [PubMed] [Google Scholar]
- Spät A., Bradford P. G., McKinney J. S., Rubin R. P., Putney J. W., Jr A saturable receptor for 32P-inositol-1,4,5-triphosphate in hepatocytes and neutrophils. Nature. 1986 Feb 6;319(6053):514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theibert A. B., Estevez V. A., Ferris C. D., Danoff S. K., Barrow R. K., Prestwich G. D., Snyder S. H. Inositol 1,3,4,5-tetrakisphosphate and inositol hexakisphosphate receptor proteins: isolation and characterization from rat brain. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3165–3169. doi: 10.1073/pnas.88.8.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theibert A. B., Supattapone S., Ferris C. D., Danoff S. K., Evans R. K., Snyder S. H. Solubilization and separation of inositol 1,3,4,5-tetrakisphosphate- and inositol 1,4,5-trisphosphate-binding proteins and metabolizing enzymes in rat brain. Biochem J. 1990 Apr 15;267(2):441–445. doi: 10.1042/bj2670441. [DOI] [PMC free article] [PubMed] [Google Scholar]


