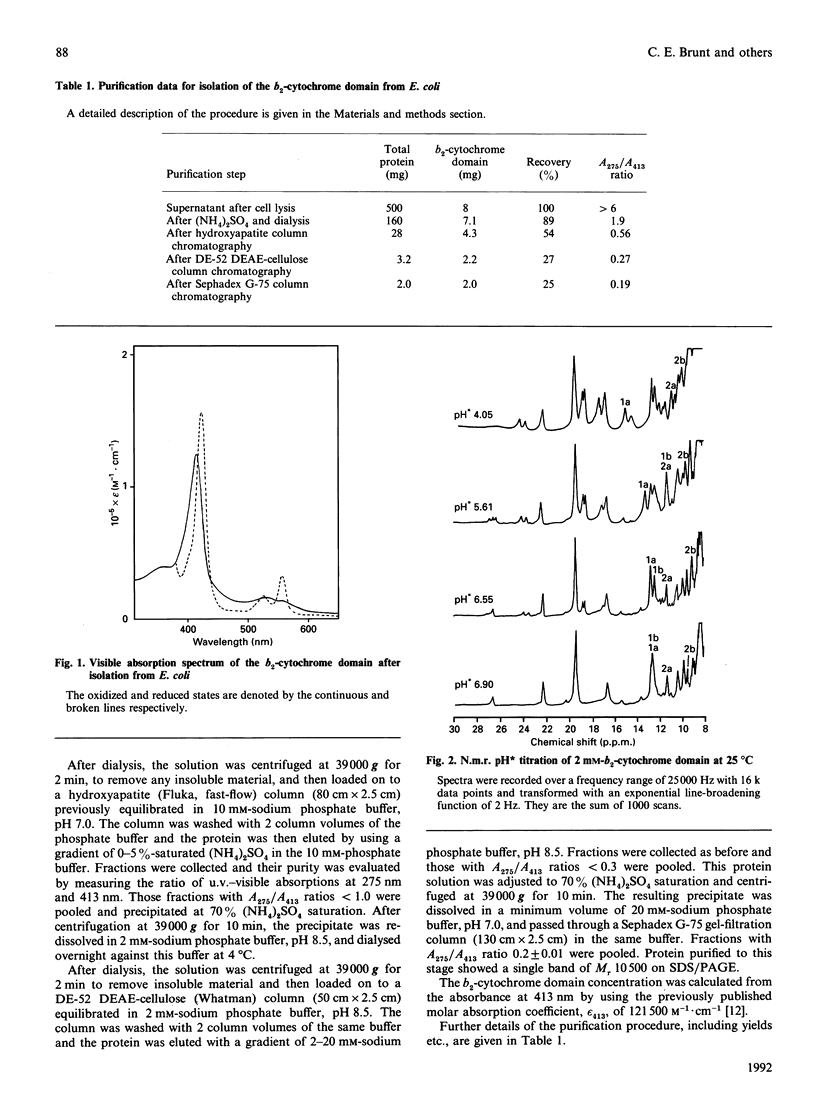
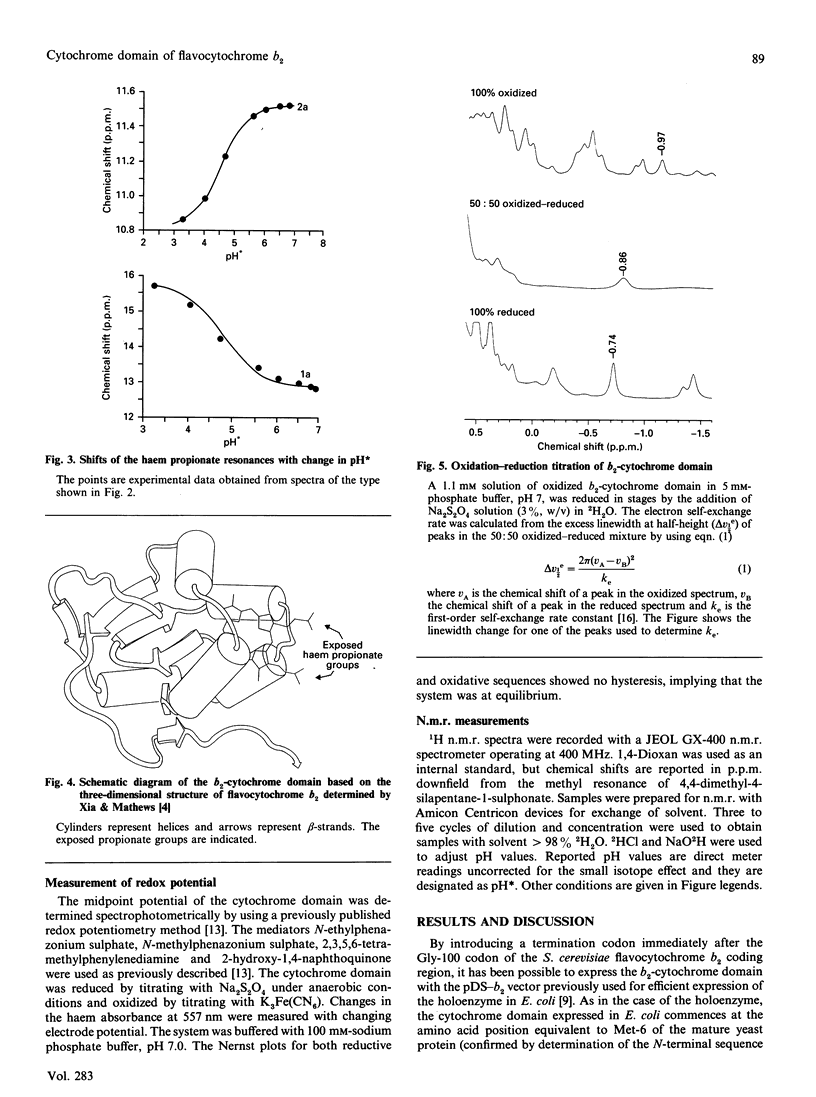
Abstract
The cytochrome domain of flavocytochrome b2 (L-lactate dehydrogenase) was expressed in the bacterium Escherichia coli and a purification procedure was developed. When expressed in E. coli, the b2-cytochrome domain contains protohaem IX and has an electronic absorption spectrum identical with that of the cytochrome b2 'core' produced by proteolytic cleavage of the enzyme isolated from yeast. The b2-cytochrome domain isolated from E. coli has an Mr of 10,500 and a redox potential of -31 +/- 2 mV. High-field n.m.r. studies indicate pKa values for the haem propionate groups to be 4.8 and 4.6, consistent with these groups being exposed to solvent rather than buried inside the protein. Using n.m.r. spectroscopy, we have determined an electron self-exchange rate constant for the b2-cytochrome domain of 2.3 x 10(6) M-1.s-1, which is more than two orders of magnitude larger than the value obtained for microsomal cytochrome b5, a homologue of b2-cytochrome domain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEBY C. A., MORTON R. K. Crystalline cytochrome b2 and lactic dehydrogenase of yeast. Nature. 1954 Apr 24;173(4408):749–752. doi: 10.1038/173749a0. [DOI] [PubMed] [Google Scholar]
- Black M. T., White S. A., Reid G. A., Chapman S. K. High-level expression of fully active yeast flavocytochrome b2 in Escherichia coli. Biochem J. 1989 Feb 15;258(1):255–259. doi: 10.1042/bj2580255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier J., Risler Y., Schwencke J., Janot J. M., Gervais M. Isolation of the flavodehydrogenase domain of Hansenula anomala flavocytochrome b2 after mild proteolysis by an H. anomala proteinase. Eur J Biochem. 1989 Jun 1;182(1):67–75. doi: 10.1111/j.1432-1033.1989.tb14801.x. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- Guiard B., Lederer F. Complete amino acid sequence of the heme-binding core in bakers' yeast cytochrome b2 (L-(+)-lactate dehydrogenase). Biochimie. 1976;58(3):305–316. doi: 10.1016/s0300-9084(76)80437-3. [DOI] [PubMed] [Google Scholar]
- Guiard B., Lederer F. The "cytochrome b5 fold": structure of a novel protein superfamily. J Mol Biol. 1979 Dec 15;135(3):639–650. doi: 10.1016/0022-2836(79)90169-4. [DOI] [PubMed] [Google Scholar]
- Jacq C., Lederer F. Cytochrome b2 from bakers' yeast (L-lactate dehydrogenase). A double-headed enzyme. Eur J Biochem. 1974 Jan 16;41(2):311–320. doi: 10.1111/j.1432-1033.1974.tb03271.x. [DOI] [PubMed] [Google Scholar]
- Keller R., Groudinsky O., Wüthrich K. Proton magnetic resonances in cytochrome b2 core. Structural similarities with cytochrome b5. Biochim Biophys Acta. 1973 Dec 6;328(2):233–238. doi: 10.1016/0005-2795(73)90256-0. [DOI] [PubMed] [Google Scholar]
- Pajot P., Groudinsky O. Molecular weight and quaternary structure of yeast L-lactate dehydrogenase (cytochrome b2). 2. Revised heme extinction coefficients and minimal molecular weight. Eur J Biochem. 1970 Jan;12(1):158–164. doi: 10.1111/j.1432-1033.1970.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Reid G. A., White S., Black M. T., Lederer F., Mathews F. S., Chapman S. K. Probing the active site of flavocytochrome b2 by site-directed mutagenesis. Eur J Biochem. 1988 Dec 15;178(2):329–333. doi: 10.1111/j.1432-1033.1988.tb14454.x. [DOI] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z. X., Mathews F. S. Molecular structure of flavocytochrome b2 at 2.4 A resolution. J Mol Biol. 1990 Apr 20;212(4):837–863. doi: 10.1016/0022-2836(90)90240-M. [DOI] [PubMed] [Google Scholar]


