Abstract
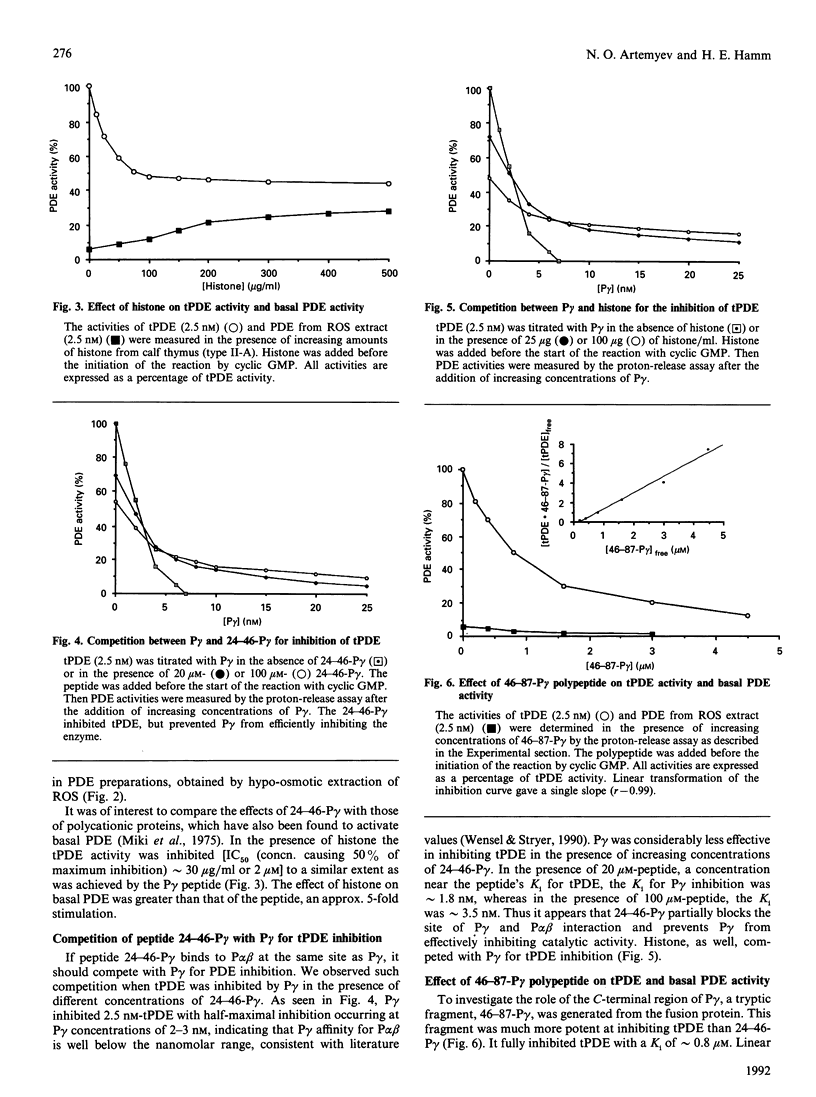
Light-activated cyclic GMP-phosphodiesterase (PDE) is the key effector enzyme of vertebrate photoreceptor cells which regulates the level of the internal transmitter cyclic GMP. PDE consists of catalytic P alpha and P beta subunits, and two copies of inhibitory P gamma subunit. The two P gamma subunits block the enzyme's activity in the dark and are removed by the alpha-subunit of transducin (alpha 1) upon light-activation of photoreceptor cells. Here we have examined the role of various regions of P gamma, the N-terminal, the central cationic and the C-terminal regions, in interaction with the catalytic subunits of PDE. N-Terminal truncation of P gamma (12-87-P gamma) did not change the potency of PDE inhibition, and thus we conclude that the P gamma N-terminal region is not critical for P gamma-P alpha beta interaction. The central region, 24-46-P gamma, participates in interaction with the catalytic P alpha beta subunits. A synthetic peptide corresponding to this site inhibited approximately 50% of trypsin-activated PDE (tPDE) (Ki approximately 15 microM) and competed with P gamma for inhibition of tPDE. We demonstrated, by using h.p.l.c. gel filtration, that 125I-Tyr-24-46-P gamma peptide bound with high affinity to tPDE, but not to P alpha beta gamma 2. The C-terminal region of 46-87-P gamma was found to be the major region involved in inhibition of PDE. It fully inhibited tPDE with a Ki of approximately 0.8 microM. It also bound to tPDE, but not P alpha beta gamma 2, in h.p.l.c. gel-filtration experiments. In addition, P gamma was cross-linked by p-phenylenedimaleimide to both P alpha and P beta, as was shown by using subunit-specific anti-P alpha, -P beta and -P gamma antibodies. Cys68 of P gamma, which presumably participates in cross-linking, is located near the P gamma C-terminus. These data provide evidence for two regions of P gamma that interact with, and inhibit, P alpha beta. The central region, 24-46 P gamma, is important in binding, but inhibits PDE only weakly, whereas the C-terminal region is most important for PDE inhibition. These results help to explain the well-known fact that P gamma trypsin-activation and C-terminal truncation both lead to PDE activation. Furthermore, our findings on the mechanism of PDE inhibition of P gamma are relevant for understanding the mechanism of PDE activation by transducin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
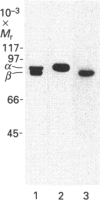
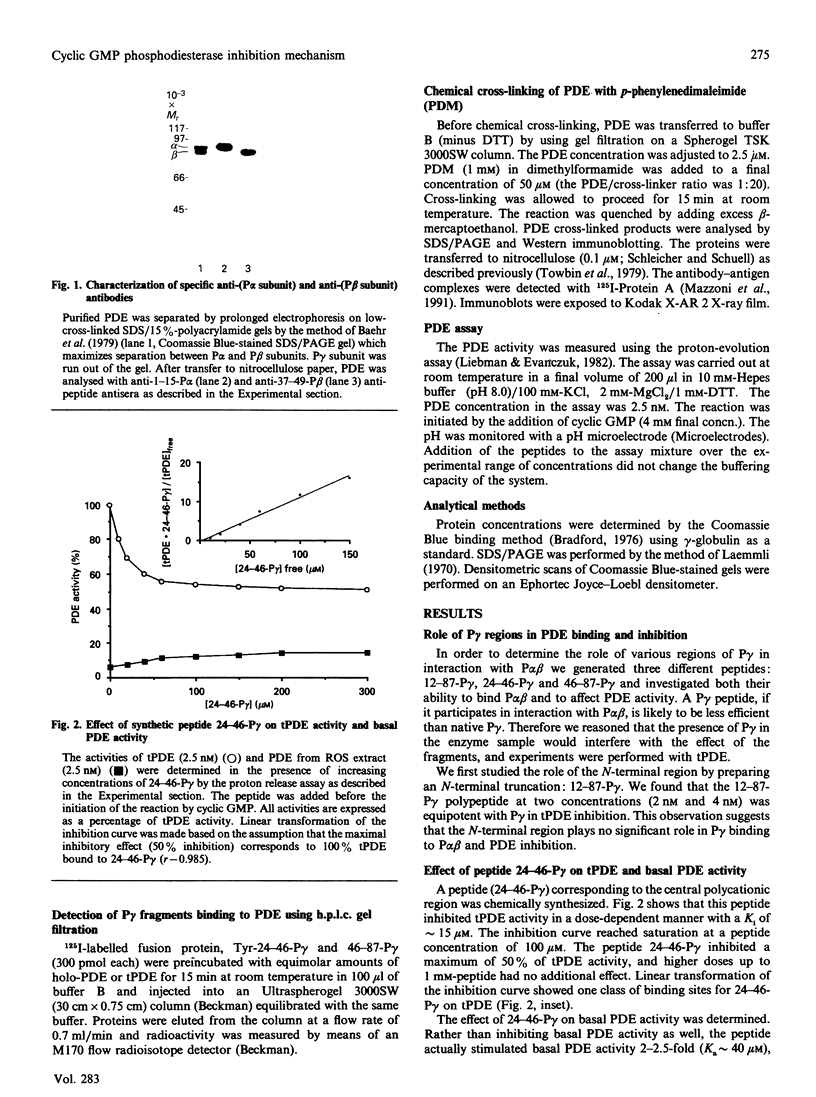
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Stryer L. Expression in bacteria of functional inhibitory subunit of retinal rod cGMP phosphodiesterase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4922–4926. doi: 10.1073/pnas.86.13.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M., Deterre P. Molecular mechanism of visual transduction. Eur J Biochem. 1989 Feb 1;179(2):255–266. doi: 10.1111/j.1432-1033.1989.tb14549.x. [DOI] [PubMed] [Google Scholar]
- Cunnick J. M., Hurt D., Oppert B., Sakamoto K., Takemoto D. J. Binding of the gamma-subunit of retinal rod-outer-segment phosphodiesterase with both transducin and the catalytic subunits of phosphodiesterase. Biochem J. 1990 Nov 1;271(3):721–727. doi: 10.1042/bj2710721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Forquet F., Robert M., Chabre M. cGMP phosphodiesterase of retinal rods is regulated by two inhibitory subunits. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2424–2428. doi: 10.1073/pnas.85.8.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Robert M., Pfister C., Kühn H., Chabre M. Activation of retinal rod cyclic GMP-phosphodiesterase by transducin: characterization of the complex formed by phosphodiesterase inhibitor and transducin alpha-subunit. Proteins. 1986 Oct;1(2):188–193. doi: 10.1002/prot.340010210. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Griswold-Prenner I. G protein-effector coupling: binding of rod phosphodiesterase inhibitory subunit to transducin. Biochemistry. 1989 Apr 18;28(8):3133–3137. doi: 10.1021/bi00434a003. [DOI] [PubMed] [Google Scholar]
- Hingorani V. N., Tobias D. T., Henderson J. T., Ho Y. K. Chemical cross-linking of bovine retinal transducin and cGMP phosphodiesterase. J Biol Chem. 1988 May 15;263(14):6916–6926. [PubMed] [Google Scholar]
- Hurley J. B. Molecular properties of the cGMP cascade of vertebrate photoreceptors. Annu Rev Physiol. 1987;49:793–812. doi: 10.1146/annurev.ph.49.030187.004045. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Stryer L. Purification and characterization of the gamma regulatory subunit of the cyclic GMP phosphodiesterase from retinal rod outer segments. J Biol Chem. 1982 Sep 25;257(18):11094–11099. [PubMed] [Google Scholar]
- Kroll S., Phillips W. J., Cerione R. A. The regulation of the cyclic GMP phosphodiesterase by the GDP-bound form of the alpha subunit of transducin. J Biol Chem. 1989 Mar 15;264(8):4490–4497. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Evanczuk A. T. Real time assay of rod disk membrane cGMP phosphodiesterase and its controller enzymes. Methods Enzymol. 1982;81:532–542. doi: 10.1016/s0076-6879(82)81074-4. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Parker K. R., Dratz E. A. The molecular mechanism of visual excitation and its relation to the structure and composition of the rod outer segment. Annu Rev Physiol. 1987;49:765–791. doi: 10.1146/annurev.ph.49.030187.004001. [DOI] [PubMed] [Google Scholar]
- Lipkin V. M., Dumler I. L., Muradov K. G., Artemyev N. O., Etingof R. N. Active sites of the cyclic GMP phosphodiesterase gamma-subunit of retinal rod outer segments. FEBS Lett. 1988 Jul 18;234(2):287–290. doi: 10.1016/0014-5793(88)80100-5. [DOI] [PubMed] [Google Scholar]
- Lipkin V. M., Khramtsov N. V., Vasilevskaya I. A., Atabekova N. V., Muradov K. G., Gubanov V. V., Li T., Johnston J. P., Volpp K. J., Applebury M. L. Beta-subunit of bovine rod photoreceptor cGMP phosphodiesterase. Comparison with the phosphodiesterase family. J Biol Chem. 1990 Aug 5;265(22):12955–12959. [PubMed] [Google Scholar]
- Lipkin V. M., Udovichenko I. P., Bondarenko V. A., Yurovskaya A. A., Telnykh E. V., Skiba N. P. Site-directed mutagenesis of the inhibitory subunit of retinal rod cyclic GMP phosphodiesterase. Biomed Sci. 1990 Mar;1(3):305–308. [PubMed] [Google Scholar]
- Mazzoni M. R., Malinski J. A., Hamm H. E. Structural analysis of rod GTP-binding protein, Gt. Limited proteolytic digestion pattern of Gt with four proteases defines monoclonal antibody epitope. J Biol Chem. 1991 Jul 25;266(21):14072–14081. [PubMed] [Google Scholar]
- McNaughton P. A. Light response of vertebrate photoreceptors. Physiol Rev. 1990 Jul;70(3):847–883. doi: 10.1152/physrev.1990.70.3.847. [DOI] [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Morrison D. F., Cunnick J. M., Oppert B., Takemoto D. J. Interaction of the gamma-subunit of retinal rod outer segment phosphodiesterase with transducin. Use of synthetic peptides as functional probes. J Biol Chem. 1989 Jul 15;264(20):11671–11681. [PubMed] [Google Scholar]
- Morrison D. F., Rider M. A., Takemoto D. J. Modulation of retinal transducin and phosphodiesterase activities by synthetic peptides of the phosphodiesterase gamma-subunit. FEBS Lett. 1987 Oct 5;222(2):266–270. doi: 10.1016/0014-5793(87)80383-6. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Gubanov V. V., Khramtsov N. V., Ischenko K. A., Zagranichny V. E., Muradov K. G., Shuvaeva T. M., Lipkin V. M. Cyclic GMP phosphodiesterase from bovine retina. Amino acid sequence of the alpha-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1987 Oct 19;223(1):169–173. doi: 10.1016/0014-5793(87)80530-6. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Lipkin V. M., Kumarev V. P., Gubanov V. V., Khramtsov N. V., Akhmedov N. B., Zagranichny V. E., Muradov K. G. Cyclic GMP phosphodiesterase from cattle retina. Amino acid sequence of the gamma-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1986 Aug 18;204(2):288–292. doi: 10.1016/0014-5793(86)80830-4. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Sitaramayya A., Harkness J., Parkes J. H., Gonzalez-Oliva C., Liebman P. A. Kinetic studies suggest that light-activated cyclic GMP phosphodiesterase is a complex with G-protein subunits. Biochemistry. 1986 Feb 11;25(3):651–656. doi: 10.1021/bi00351a021. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensel T. G., Stryer L. Activation mechanism of retinal rod cyclic GMP phosphodiesterase probed by fluorescein-labeled inhibitory subunit. Biochemistry. 1990 Feb 27;29(8):2155–2161. doi: 10.1021/bi00460a028. [DOI] [PubMed] [Google Scholar]
- Wensel T. G., Stryer L. Reciprocal control of retinal rod cyclic GMP phosphodiesterase by its gamma subunit and transducin. Proteins. 1986 Sep;1(1):90–99. doi: 10.1002/prot.340010114. [DOI] [PubMed] [Google Scholar]
- Yamazaki A., Hayashi F., Tatsumi M., Bitensky M. W., George J. S. Interactions between the subunits of transducin and cyclic GMP phosphodiesterase in Rana catesbiana rod photoreceptors. J Biol Chem. 1990 Jul 15;265(20):11539–11548. [PubMed] [Google Scholar]