Abstract
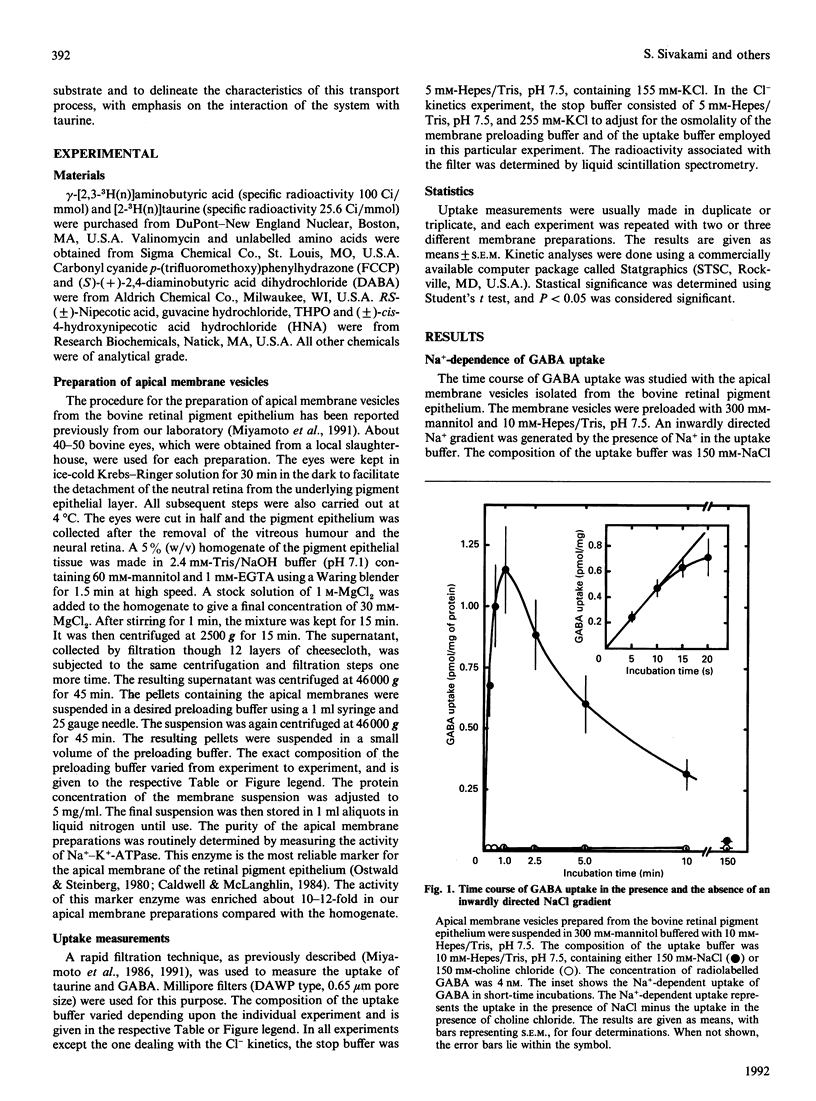
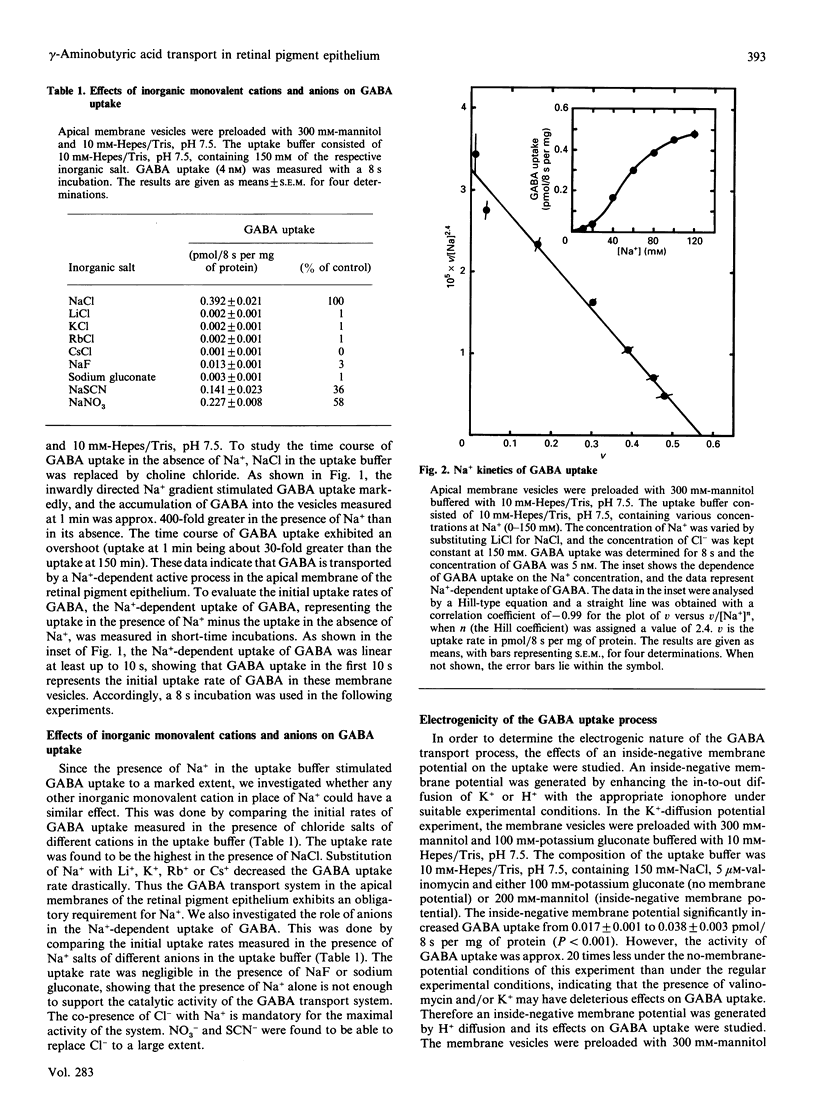
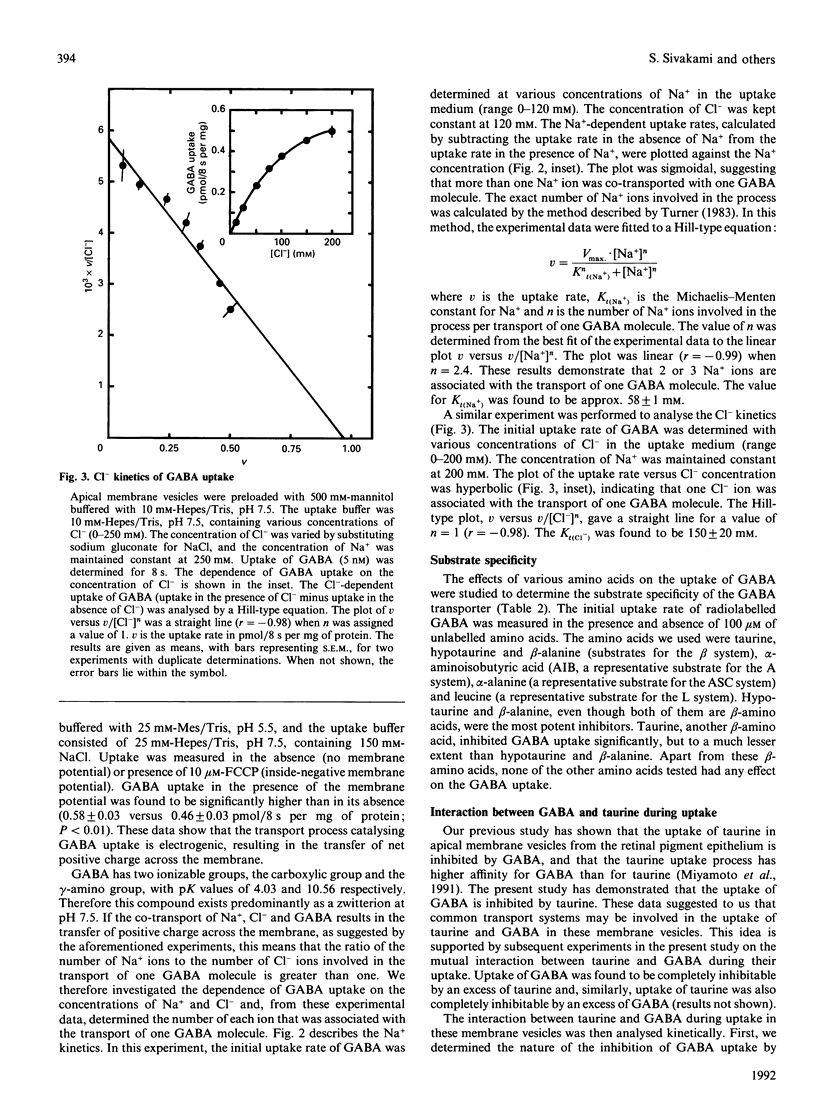
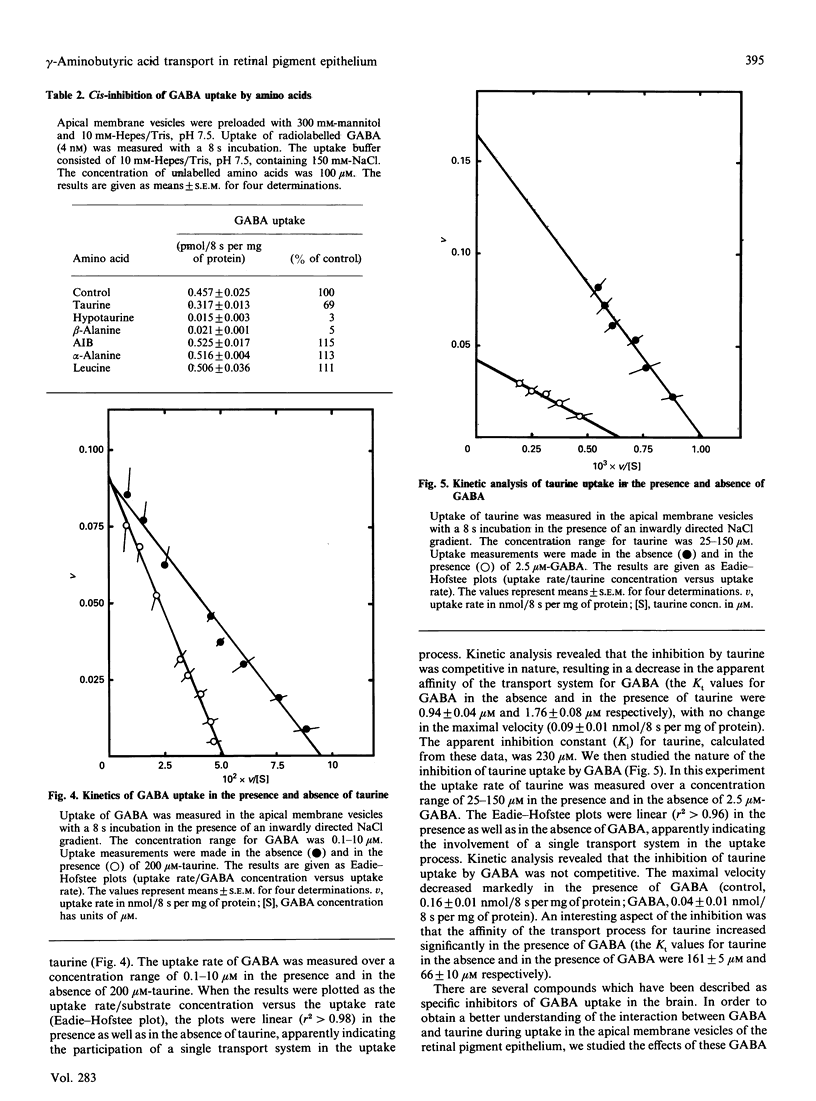
The characteristics of gamma-aminobutyric acid (GABA) uptake were investigated in apical membrane vesicles prepared from the bovine retinal pigment epithelium. An inwardly directed NaCl gradient stimulated GABA uptake markedly, and the time course of uptake exhibited an overshoot phenomenon indicating the presence of an active transport mechanism for GABA in these membranes. Other monovalent cations were not capable of substituting for Na+. In addition to this obligatory requirement for Na+, the GABA uptake also exhibited a Cl(-)-dependence, evident from the observations that the uptake was negligible in the presence of NaF or sodium gluconate in place of NaCl. NO3- and SCN- could substitute for Cl- to some extent. The uptake process was electrogenic, with a Na+/Cl-/GABA stoichiometry of 2:1:1 or 3:1:1. Substrate-specificity studies showed that the beta-amino acids such as taurine, hypotaurine and beta-alanine interacted with the GABA uptake process. Uptake of GABA could be completely inhibited by an excess of taurine and, similarly, uptake of taurine could be completely inhibited by an excess of GABA, suggesting that common transport processes operate in the uptake of these two compounds. However, a number of compounds which are specific inhibitors of GABA uptake inhibited taurine uptake only to a maximum of 50%. Kinetic analysis of GABA uptake in the concentration range 0.1-10 microM revealed that the uptake occurred via a single system and that taurine was a competitive inhibitor of this system. The Michaelis-Menten constant (Kt) for GABA was 0.94 microM and the apparent inhibition constant (Ki) for taurine was 230 microM. On the contrary, even though the kinetic analysis of taurine uptake in the concentration range 25-150 microM revealed participation of a single system in the uptake process, the inhibition of taurine uptake by GABA was not competitive. The presence of GABA decreased the maximal velocity of the taurine uptake process and also decreased the Kt for taurine. Based on these data, it is proposed that: (i) there are two distinct transport systems, namely the GABA transporter and the taurine transporter, in these membranes which accept both GABA and taurine as substrates, (ii) the affinities of these systems for taurine are very similar and cannot be kinetically distinguished under the experimental conditions employed, and (iii) the difference between the affinities of these system for GABA is much greater than for taurine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard J. A., Thaxter S., Kikuchi K., Ghishan F. K. Taurine transport by rat intestine. Am J Physiol. 1988 Mar;254(3 Pt 1):G334–G338. doi: 10.1152/ajpgi.1988.254.3.G334. [DOI] [PubMed] [Google Scholar]
- Caldwell R. B., McLaughlin B. J. Redistribution of Na-K-ATPase in the dystrophic rat retinal pigment epithelium. J Neurocytol. 1984 Dec;13(6):895–910. doi: 10.1007/BF01148592. [DOI] [PubMed] [Google Scholar]
- Edwards R. B. Accumulation of taurine by cultured retinal pigment epithelium of the rat. Invest Ophthalmol Vis Sci. 1977 Mar;16(3):201–208. [PubMed] [Google Scholar]
- Kanner B. I. Active transport of gamma-aminobutyric acid by membrane vesicles isolated from rat brain. Biochemistry. 1978 Apr 4;17(7):1207–1211. doi: 10.1021/bi00600a011. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Bendahan A. Two pharmacologically distinct sodium- and chloride-coupled high-affinity gamma-aminobutyric acid transporters are present in plasma membrane vesicles and reconstituted preparations from rat brain. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2550–2554. doi: 10.1073/pnas.87.7.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl P. I., Fisher S. E. Taurine transport by microvillous membrane vesicles and the perfused cotyledon of the human placenta. Am J Physiol. 1990 Mar;258(3 Pt 1):C443–C451. doi: 10.1152/ajpcell.1990.258.3.C443. [DOI] [PubMed] [Google Scholar]
- Kulanthaivel P., Leibach F. H., Mahesh V. B., Ganapathy V. Tyrosine residues are essential for the activity of the human placental taurine transporter. Biochim Biophys Acta. 1989 Oct 16;985(2):139–146. doi: 10.1016/0005-2736(89)90358-1. [DOI] [PubMed] [Google Scholar]
- Marshall J., Voaden M. Autoradiographic identification of the cells accumulating 3H gamma-aminobutyric acid in mammalian retinae: a species comparison. Vision Res. 1975 Mar;15(3):459–461. doi: 10.1016/0042-6989(75)90102-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Balkovetz D. F., Leibach F. H., Mahesh V. B., Ganapathy V. Na+ + Cl- -gradient-driven, high-affinity, uphill transport of taurine in human placental brush-border membrane vesicles. FEBS Lett. 1988 Apr 11;231(1):263–267. doi: 10.1016/0014-5793(88)80744-0. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Ganapathy V., Leibach F. H. Identification of histidyl and thiol groups at the active site of rabbit renal dipeptide transporter. J Biol Chem. 1986 Dec 5;261(34):16133–16140. [PubMed] [Google Scholar]
- Miyamoto Y., Kulanthaivel P., Leibach F. H., Ganapathy V. Taurine uptake in apical membrane vesicles from the bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1991 Aug;32(9):2542–2551. [PubMed] [Google Scholar]
- Miyamoto Y., Tiruppathi C., Ganapathy V., Leibach F. H. Active transport of taurine in rabbit jejunal brush-border membrane vesicles. Am J Physiol. 1989 Jul;257(1 Pt 1):G65–G72. doi: 10.1152/ajpgi.1989.257.1.G65. [DOI] [PubMed] [Google Scholar]
- Ostwald T. J., Steinberg R. H. Localization of frog retinal pigment epithelium Na+-K+ ATPase. Exp Eye Res. 1980 Sep;31(3):351–360. doi: 10.1016/s0014-4835(80)80043-1. [DOI] [PubMed] [Google Scholar]
- Ostwald T. J., Steinberg R. H. Transmembrane components of taurine flux across frog retinal pigment epithelium. Curr Eye Res. 1981;1(8):437–443. doi: 10.3109/02713688109019983. [DOI] [PubMed] [Google Scholar]
- Pastuszko A., Wilson D. F., Erecinska M. Energetics of gamma-aminobutyrate transport in rat brain synaptosomes. J Biol Chem. 1982 Jul 10;257(13):7514–7519. [PubMed] [Google Scholar]
- Radian R., Kanner B. I. Stoichiometry of sodium- and chloride-coupled gamma-aminobutyric acid transport by synaptic plasma membrane vesicles isolated from rat brain. Biochemistry. 1983 Mar 1;22(5):1236–1241. doi: 10.1021/bi00274a038. [DOI] [PubMed] [Google Scholar]
- Turner R. J. Quantitative studies of cotransport systems: models and vesicles. J Membr Biol. 1983;76(1):1–15. doi: 10.1007/BF01871450. [DOI] [PubMed] [Google Scholar]
- Wolff N. A., Kinne R. Taurine transport by rabbit kidney brush-border membranes: coupling to sodium, chloride, and the membrane potential. J Membr Biol. 1988 May;102(2):131–139. doi: 10.1007/BF01870451. [DOI] [PubMed] [Google Scholar]
- Zelikovic I., Stejskal-Lorenz E., Lohstroh P., Budreau A., Chesney R. W. Anion dependence of taurine transport by rat renal brush-border membrane vesicles. Am J Physiol. 1989 Apr;256(4 Pt 2):F646–F655. doi: 10.1152/ajprenal.1989.256.4.F646. [DOI] [PubMed] [Google Scholar]


