Abstract
The permeability properties of a putative Ca2(+)-activated pore in heart mitochondria, of possible relevance to re-perfusion-induced injury, have been investigated by a pulsed-flow solute-entrapment technique. The relative permeabilities of [14C]mannitol, [14C]sucrose and arsenazo III are consistent with permeation via a pore of about 2.3 nm diameter. Ca2+ removal with EGTA induced pore closure, and the mitochondria became 'resealed'. The permeability of the unresealed mitochondria during resealing was markedly stimulated by 200 microM-ADP, and the relative permeabilities to solutes of different size were stimulated equally, indicating an increase in open-pore number, rather than an increase in pore dimensions. This is paradoxical, since ADP also stimulated the rate of resealing. The rate of EGTA-induced resealing was also stimulated by the Ca2+ ionophore A23187, which indicates that the rate of removal of matrix free Ca2+ is limiting for pore closure. An explanation for the paradox is suggested in which ADP facilitates pore interconversion between the closed and open states in permeabilized mitochondria, and pore closure in Ca2(+)-free mitochondria occurs much faster than previously thought.
Full text
PDF



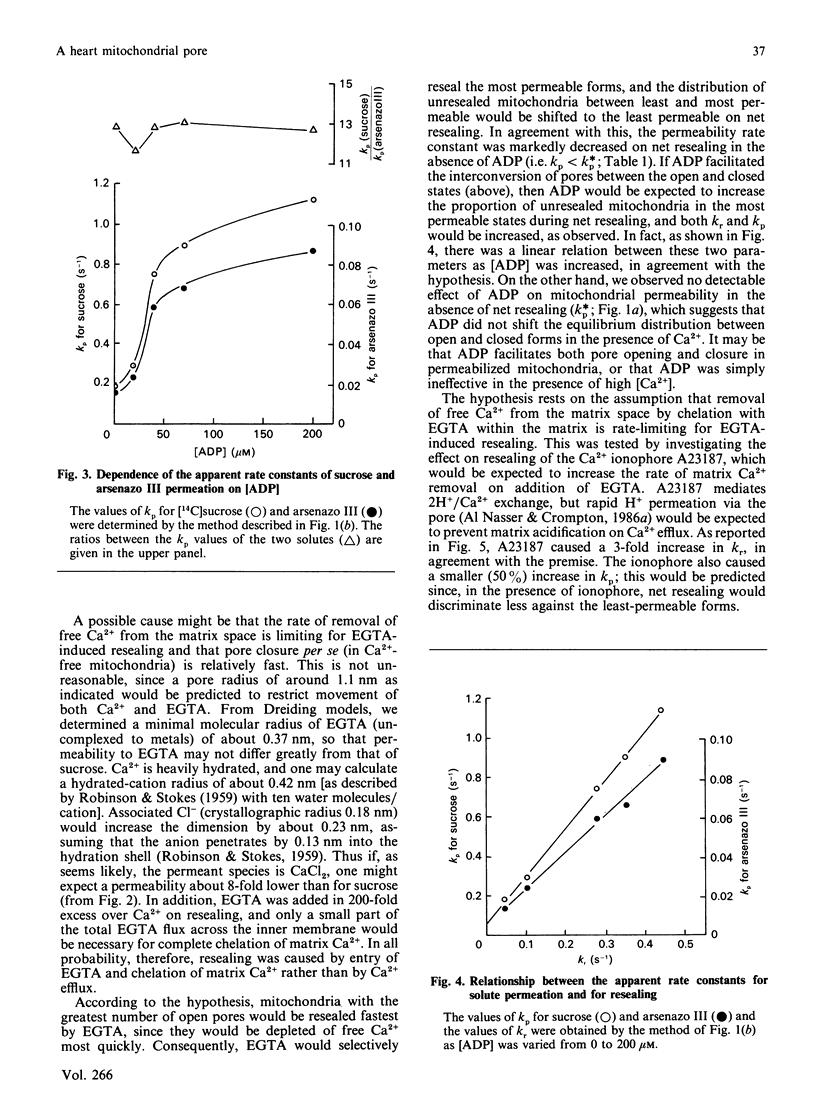
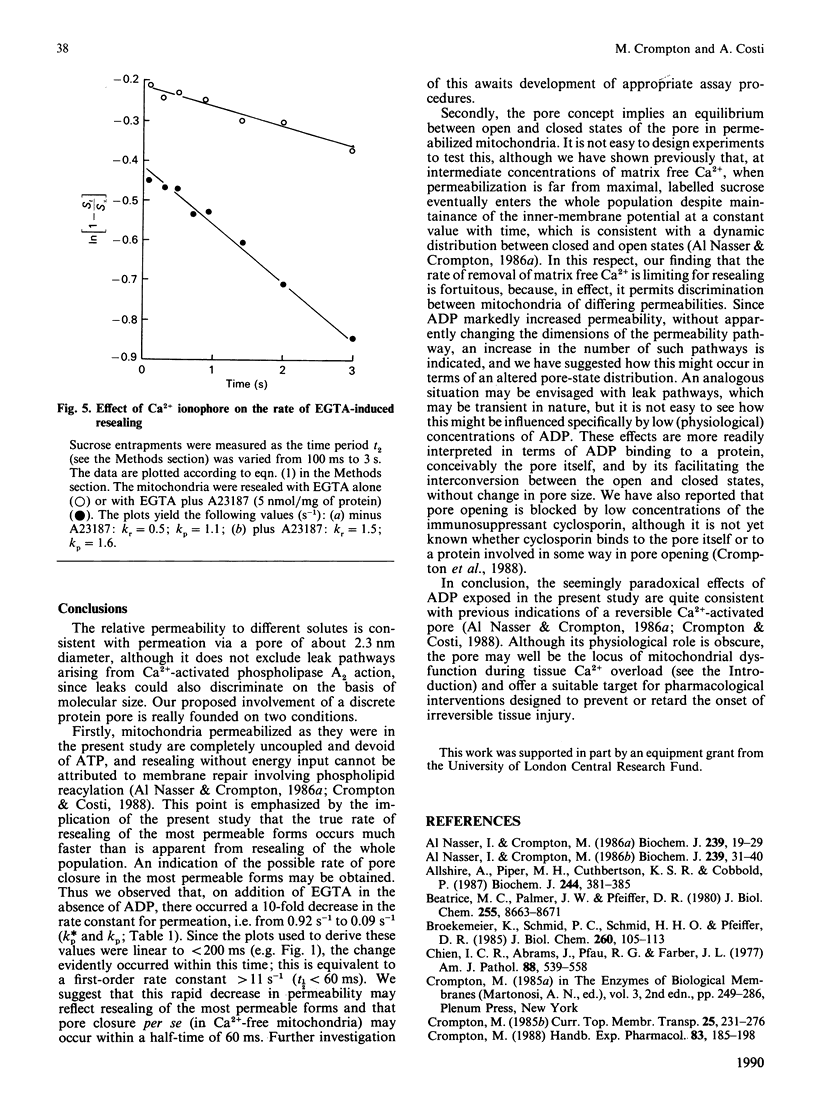

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Nasser I., Crompton M. The entrapment of the Ca2+ indicator arsenazo III in the matrix space of rat liver mitochondria by permeabilization and resealing. Na+-dependent and -independent effluxes of Ca2+ in arsenazo III-loaded mitochondria. Biochem J. 1986 Oct 1;239(1):31–40. doi: 10.1042/bj2390031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire A., Piper H. M., Cuthbertson K. S., Cobbold P. H. Cytosolic free Ca2+ in single rat heart cells during anoxia and reoxygenation. Biochem J. 1987 Jun 1;244(2):381–385. doi: 10.1042/bj2440381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatrice M. C., Palmer J. W., Pfeiffer D. R. The relationship between mitochondrial membrane permeability, membrane potential, and the retention of Ca2+ by mitochondria. J Biol Chem. 1980 Sep 25;255(18):8663–8671. [PubMed] [Google Scholar]
- Broekemeier K. M., Schmid P. C., Schmid H. H., Pfeiffer D. R. Effects of phospholipase A2 inhibitors on ruthenium red-induced Ca2+ release from mitochondria. J Biol Chem. 1985 Jan 10;260(1):105–113. [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Pfau R. G., Farber J. L. Prevention by chlorpromazine of ischemic liver cell death. Am J Pathol. 1977 Sep;88(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Costi A., Hayat L. Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J. 1987 Aug 1;245(3):915–918. doi: 10.1042/bj2450915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Costi A. Kinetic evidence for a heart mitochondrial pore activated by Ca2+, inorganic phosphate and oxidative stress. A potential mechanism for mitochondrial dysfunction during cellular Ca2+ overload. Eur J Biochem. 1988 Dec 15;178(2):489–501. doi: 10.1111/j.1432-1033.1988.tb14475.x. [DOI] [PubMed] [Google Scholar]
- Crompton M., Ellinger H., Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988 Oct 1;255(1):357–360. [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- GREENAWALT J. W., ROSSI C. S., LEHNINGER A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol. 1964 Oct;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Stein W. D. Biophysical analysis of novel transport pathways induced in red blood cell membranes. J Membr Biol. 1987;96(1):1–10. doi: 10.1007/BF01869329. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R. Allosteric inhibition of the Ca2+-activated hydrophilic channel of the mitochondrial inner membrane by nucleotides. J Membr Biol. 1980 Jun 15;54(3):231–236. doi: 10.1007/BF01870239. [DOI] [PubMed] [Google Scholar]
- Henry P. D., Schuchleib R., Davis J., Weiss E. S., Sobel B. E. Myocardial contracture and accumulation of mitochondrial calcium in ischemic rabbit heart. Am J Physiol. 1977 Dec;233(6):H677–H684. doi: 10.1152/ajpheart.1977.233.6.H677. [DOI] [PubMed] [Google Scholar]
- Lochner A., Opie L. H., Owen P., Kotzé J. C., Bruyneel K., Gevers W. Oxidative phosphorylation in infarcting baboon and dog myocardium: effects of mitochondrial isolation and incubation media. J Mol Cell Cardiol. 1975 Mar;7(3):203–217. doi: 10.1016/0022-2828(75)90159-5. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed Proc. 1987 May 15;46(7):2402–2406. [PubMed] [Google Scholar]
- Peng C. F., Kane J. J., Straub K. D., Murphy M. L. Improvement of mitochondrial energy production in ischemic myocardium by in vivo infusion of ruthenium red. J Cardiovasc Pharmacol. 1980 Jan-Feb;2(1):45–54. doi: 10.1097/00005344-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson P. A., Harding D. P., Bourdillon P. D., Tones M. A. Calcium out of control. J Mol Cell Cardiol. 1984 Feb;16(2):175–187. doi: 10.1016/s0022-2828(84)80706-3. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- ROSSI C. S., LEHNINGER A. L. STOICHIOMETRY OF RESPIRATORY STIMULATION, ACCUMULATION OF CA++ AND PHOSPHATE, AND OXIDATIVE PHOSPHORYLATION IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1964 Nov;239:3971–3980. [PubMed] [Google Scholar]
- Riley W. W., Jr, Pfeiffer D. R. The effect of Ca2+ and acyl coenzyme A:lysophospholipid acyltransferase inhibitors on permeability properties of the liver mitochondrial inner membrane. J Biol Chem. 1986 Oct 25;261(30):14018–14024. [PubMed] [Google Scholar]


