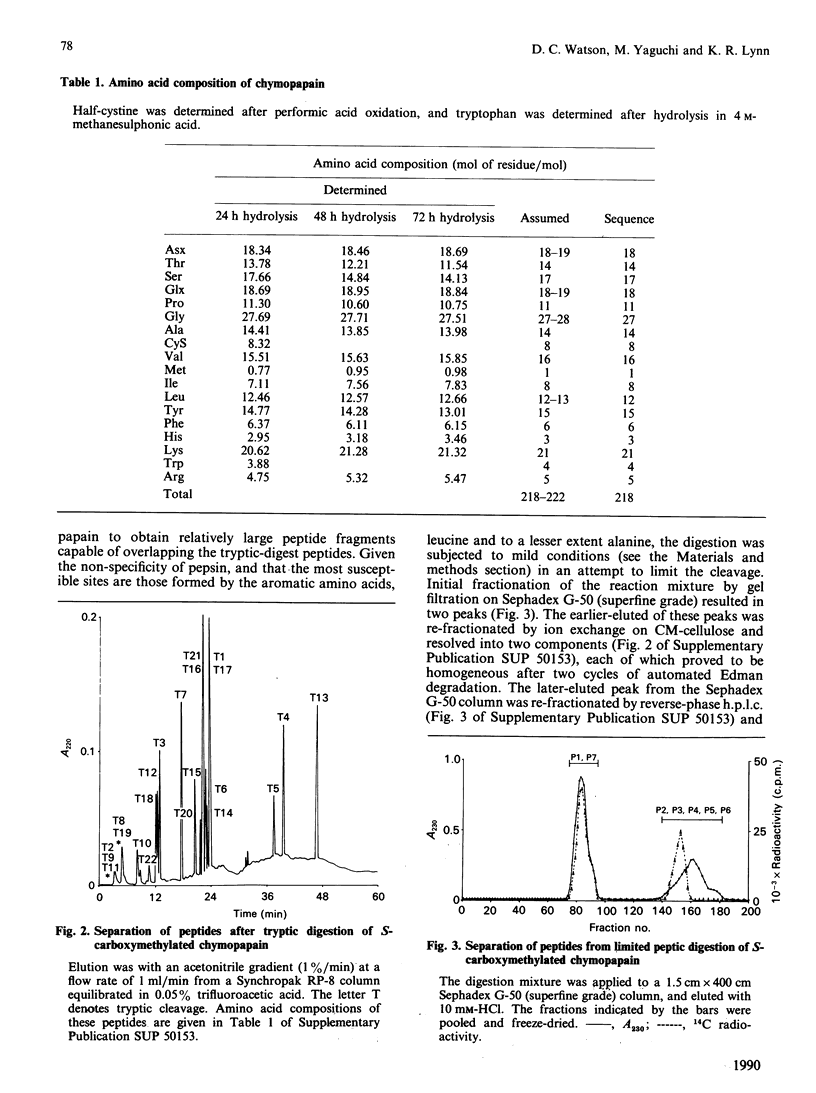
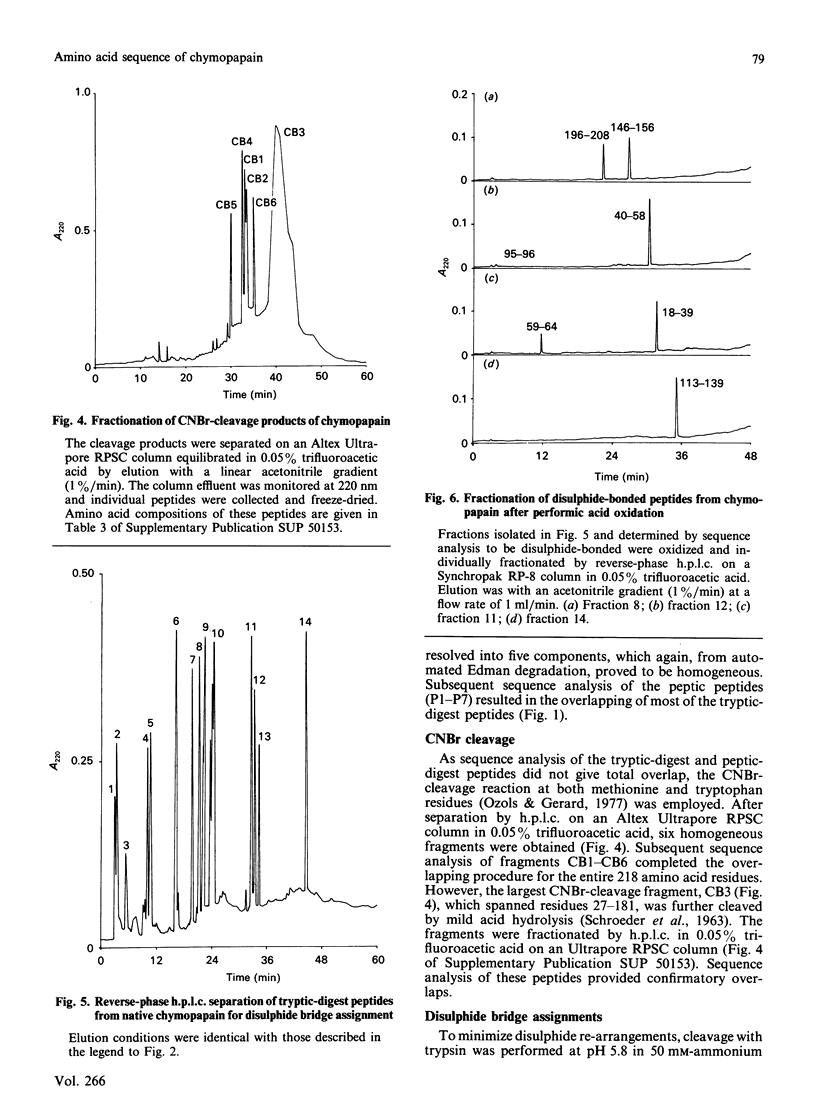
Abstract
Chymopapain is a polypeptide of 218 amino acid residues. It has considerable structural similarity with papain and papaya proteinase omega, including conservation of the catalytic site and of the disulphide bonding. Chymopapain is like papaya proteinase omega in carrying four extra residues between papain positions 168 and 169, but differs from both papaya proteinases in the composition of its S2 subsite, as well as in having a second thiol group, Cys-117. Some evidence for the amino acid sequence of chymopapain has been deposited as Supplementary Publication SUP 50153 (12 pages) at the British Library Document Supply Centre, Boston Spa., Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies may be obtained on the terms indicated in Biochem. J. (1990) 265, 5. The information comprises Supplement Tables 1-4, which contain, in order, amino acid compositions of peptides from tryptic, peptic, CNBr and mild acid cleavages, Supplement Fig. 1, showing re-fractionation of selected peaks from Fig. 2 of the main paper. Supplement Fig. 2, showing cation-exchange chromatography of the earliest-eluted peak of Fig. 3 of the main paper, Supplement Fig. 3, showing reverse-phase h.p.l.c. of the later-eluted peak from Fig. 3 of the main paper, and Supplement Fig. 4, showing the separation of peptides after mild acid hydrolysis of CNBr-cleavage fragment CB3.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker E. N. Structure of actinidin, after refinement at 1.7 A resolution. J Mol Biol. 1980 Aug 25;141(4):441–484. doi: 10.1016/0022-2836(80)90255-7. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Barrett A. J. Chymopapain. Chromatographic purification and immunological characterization. Biochem J. 1984 Oct 1;223(1):81–88. doi: 10.1042/bj2230081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carne A., Moore C. H. The amino acid sequence of the tryptic peptides from actinidin, a proteolytic enzyme from the fruit of Actinidia chinensis. Biochem J. 1978 Jul 1;173(1):73–83. doi: 10.1042/bj1730073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. W., Coghlan V. M., Dihel L. C. Cloning and sequencing of papain-encoding cDNA. Gene. 1986;48(2-3):219–227. doi: 10.1016/0378-1119(86)90080-6. [DOI] [PubMed] [Google Scholar]
- Drenth J., Kalk K. H., Swen H. M. Binding of chloromethyl ketone substrate analogues to crystalline papain. Biochemistry. 1976 Aug 24;15(17):3731–3738. doi: 10.1021/bi00662a014. [DOI] [PubMed] [Google Scholar]
- Dubois T., Kleinschmidt T., Schnek A. G., Looze Y., Braunitzer G. The thiol proteinases from the latex of Carica papaya L. II. The primary structure of proteinase omega. Biol Chem Hoppe Seyler. 1988 Aug;369(8):741–754. doi: 10.1515/bchm3.1988.369.2.741. [DOI] [PubMed] [Google Scholar]
- EBATA M., YASUNOBU K. T. Chymopapain. I. Isolation, crystallization, and preliminary characterization. J Biol Chem. 1962 Apr;237:1086–1094. [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Kalk K. H., Swarte M. B., Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233–256. doi: 10.1016/0022-2836(84)90467-4. [DOI] [PubMed] [Google Scholar]
- Kunimitsu D. K., Yasunobu K. T. Chymopapain. IV. The chromatographic fractionation of partially purified chymopapain and the characterization of crystalline chymopapain B. Biochim Biophys Acta. 1967 Jul 11;139(2):405–417. doi: 10.1016/0005-2744(67)90044-7. [DOI] [PubMed] [Google Scholar]
- Lynn K. R. A purification and some properties of two proteases from papaya latex. Biochim Biophys Acta. 1979 Aug 15;569(2):193–201. doi: 10.1016/0005-2744(79)90054-8. [DOI] [PubMed] [Google Scholar]
- Ozols J., Gerard C. Cleavage of tryptophanyl peptide bonds in cytochrome b5 by cyanogen bromide. J Biol Chem. 1977 Sep 10;252(17):5986–5989. [PubMed] [Google Scholar]
- SCHROEDER W. A., SHELTON J. R., SHELTON J. B., CORMICK J., JONES R. T. THE AMINO ACID SEQUENCE OF THE GAMMA CHAIN OF HUMAN FETAL HEMOGLOBIN. Biochemistry. 1963 Sep-Oct;2:992–1008. doi: 10.1021/bi00905a016. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A., de Graaf M. J. The fluorescence of papain. Biochim Biophys Acta. 1970 Mar 31;200(3):595–597. doi: 10.1016/0005-2795(70)90123-6. [DOI] [PubMed] [Google Scholar]
- Titani K., Sasagawa T., Resing K., Walsh K. A. A simple and rapid purification of commercial trypsin and chymotrypsin by reverse-phase high-performance liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):408–412. doi: 10.1016/0003-2697(82)90465-1. [DOI] [PubMed] [Google Scholar]
- Tsunoda J. N., Yasunobu K. T. The amino acid sequence around the reactive thiol group of chymopapain B. J Biol Chem. 1966 Oct 25;241(20):4610–4615. [PubMed] [Google Scholar]


