Abstract
The CD9 molecule is a 24 kDa surface-membrane glycoprotein present on platelets and a variety of haematopoetic and non-haematopoetic tissues. In the present study we utilized specific inhibitors of thromboxane A2 (TxA2) formation (aspirin), protein kinase C [H-7 [1-(5-isoquinolinesulphonyl)-2-methylpiperazine]] and autocrine stimulation by secreted ADP (apyrase) to modify platelet activation by a monoclonal antibody ALB-6 to the CD9 antigen. This activation is only partially inhibited by aspirin alone but, in combination with either H-7 or apyrase, more than 50% inhibition of platelet aggregation and secretion was observed. This combination of inhibitors was also required to inhibit effectively the phosphorylation of myosin light chain and the 47 kDa substrate of protein kinase C. Intracellular Ca2+ flux monitored by the fluorescent dye fura-2 showed that this was almost completely mediated by the aspirin-sensitive TxA2 pathway. We suggest that the aspirin-insensitive pathway is primarily mediated by phospholipase C formation of diacylglycerol to activate protein kinase C. The inhibition by apyrase suggests a strong dependency on autocrine stimulation by secreted ADP to fully activate both phospholipase C and express fibrinogen-binding sites mediating platelet aggregation. This alternate pathway of phospholipase C activation by ALB-6 may be mediated by cytoplasmic alkalinization [monitored by SNARF-1 (5'(6')-carboxy-10-bismethylamino-3-hydroxy-spiro-[7H- benzo[c]xanthine-1',7(3H)-isobenzofuran]-3'-one) fluorescence of the dye]. Both activation pathways are dependent on intact antibodies, since F(ab')2 fragments of SYB-1, a monoclonal antibody against the CD9 antigen with activation characteristics identical with those of ALB-6, do not elicit activation. Besides thrombin, collagen is another physiological agonist shown to induce aspirin-insensitive activation. Similarities to ALB-6 in collagen sensitivity to apyrase in combination with aspirin inhibitors were noted with respect to aggregation and secretion, as well as a complete block of Ca2+ flux by aspirin. However, it is unlikely that collagen activation is mediated by the CD9 antigen, since SYB-1 F(ab')2 fragments had no effect on collagen activation and aspirin also completely blocked the alkalinization response to collagen, in contrast with ALB-6.
Full text
PDF


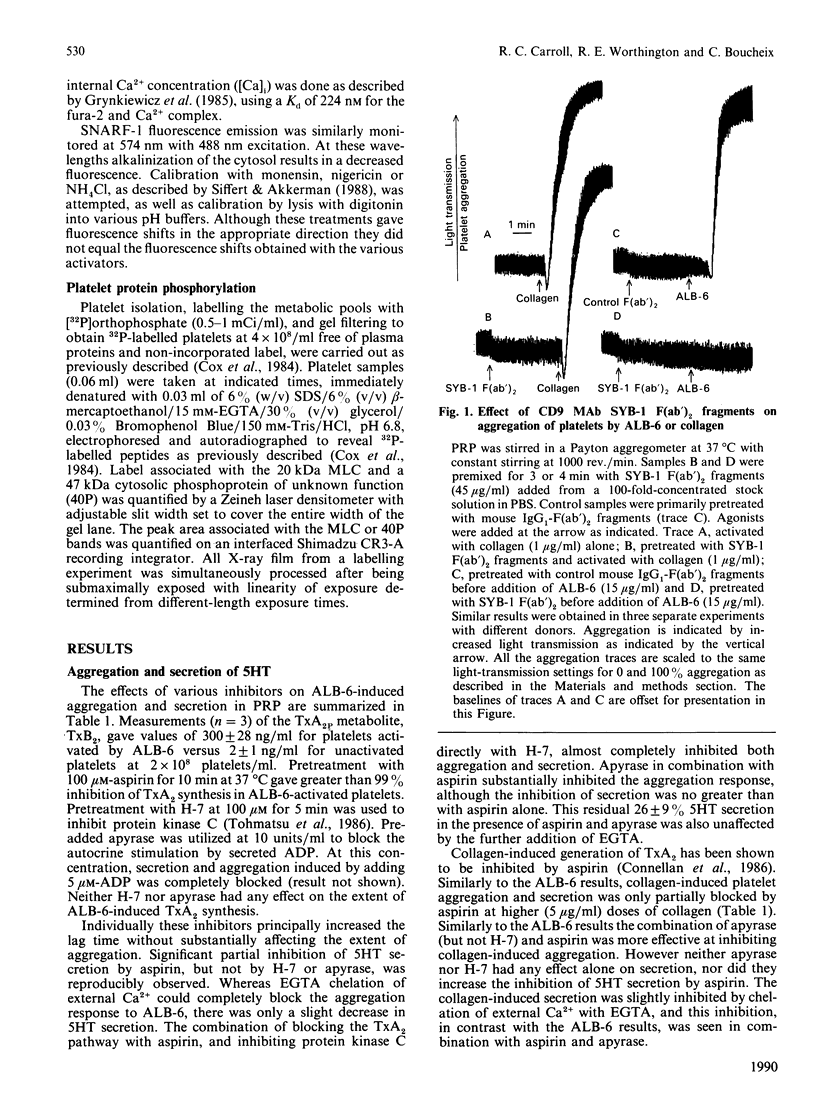
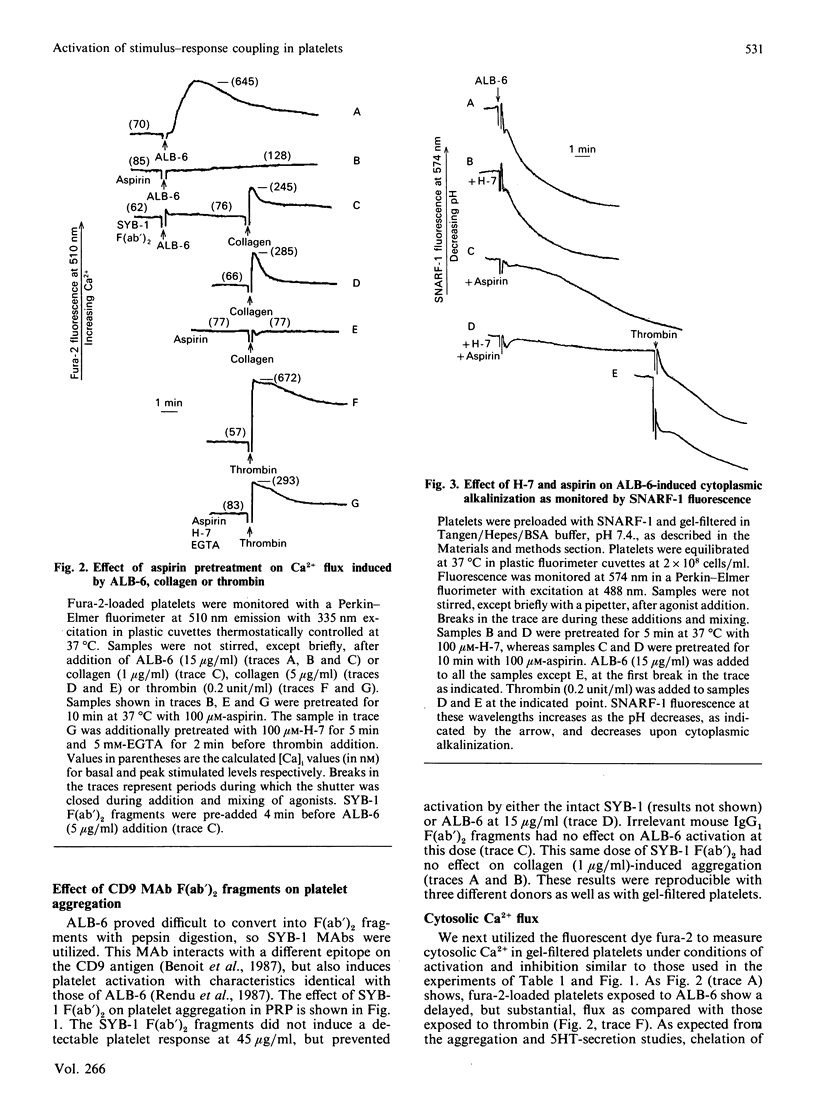
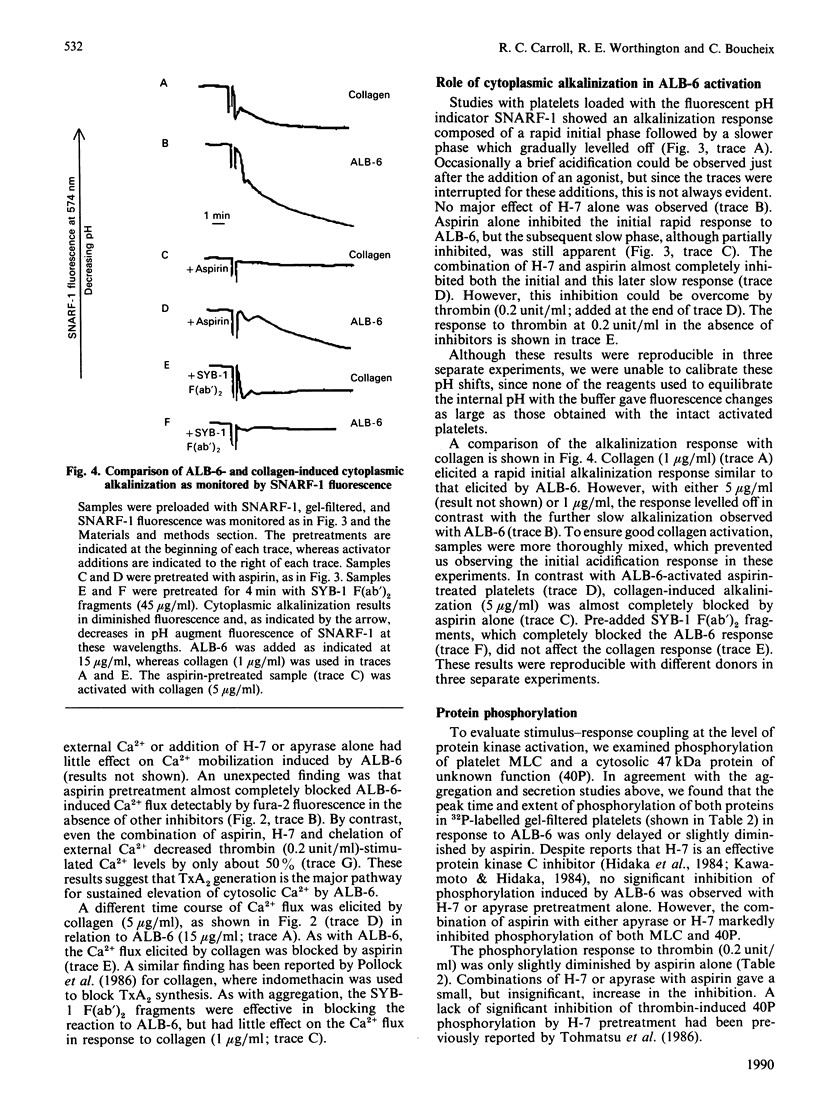
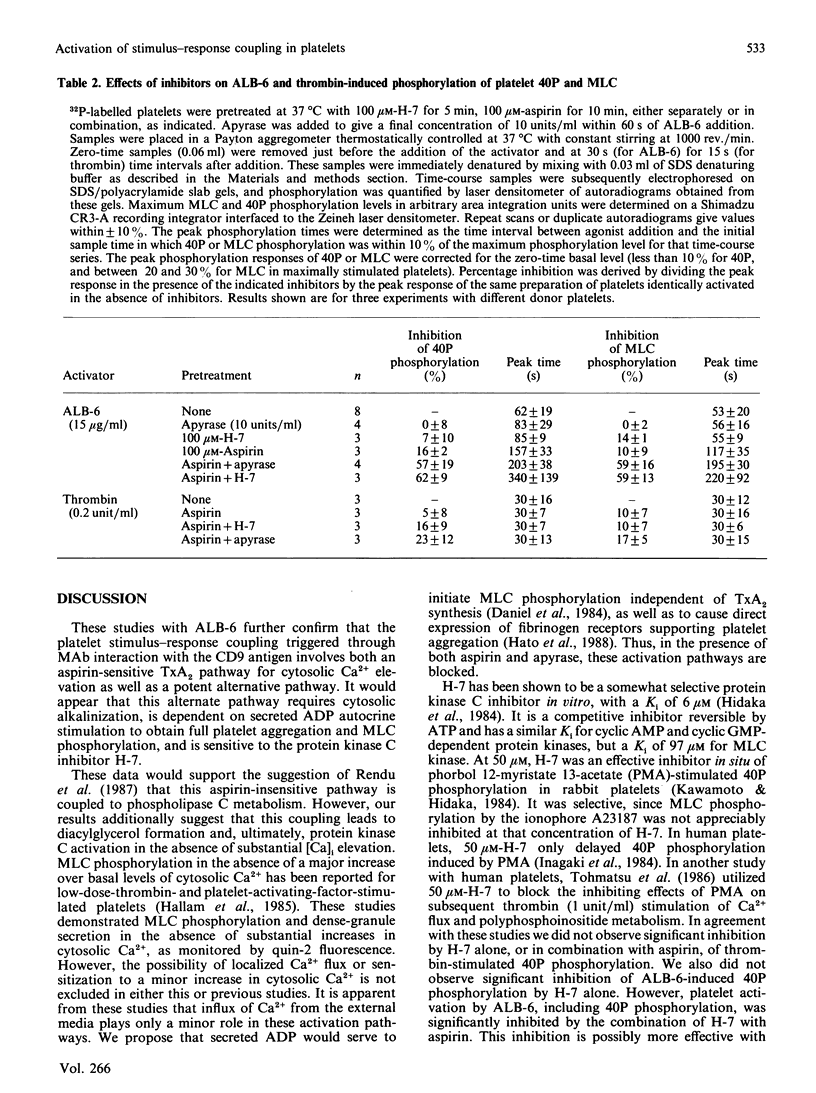


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banga H. S., Simons E. R., Brass L. F., Rittenhouse S. E. Activation of phospholipases A and C in human platelets exposed to epinephrine: role of glycoproteins IIb/IIIa and dual role of epinephrine. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9197–9201. doi: 10.1073/pnas.83.23.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucheix C., Soria C., Mirshahi M., Soria J., Perrot J. Y., Fournier N., Billard M., Rosenfeld C. Characteristics of platelet aggregation induced by the monoclonal antibody ALB6 (acute lymphoblastic leukemia antigen p 24). Inhibition of aggregation by ALB6Fab. FEBS Lett. 1983 Sep 19;161(2):289–295. doi: 10.1016/0014-5793(83)81027-8. [DOI] [PubMed] [Google Scholar]
- Chiang T. M., Jin A., Kang A. H. Platelet-collagen interaction. Inhibition by a monoclonal antibody raised against collagen receptor. J Immunol. 1987 Aug 1;139(3):887–892. [PubMed] [Google Scholar]
- Connellan J. M., Thurlow P. J., Barlow B., Lowe M., McKenzie I. F. Investigation of alternative mechanisms of collagen-induced platelet activation by using monoclonal antibodies to glycoprotein IIb-IIIa and fibrinogen. Thromb Haemost. 1986 Apr 30;55(2):153–157. [PubMed] [Google Scholar]
- Cox A. C., Carroll R. C. The effect of tetradecanoylphorbol acetate on calcium-ion mobilization, protein phosphorylation and cytoskeletal assembly induced by thrombin or arachidonate. Biochim Biophys Acta. 1986 May 29;886(3):390–398. doi: 10.1016/0167-4889(86)90174-6. [DOI] [PubMed] [Google Scholar]
- Cox A. C., Carroll R. C., White J. G., Rao G. H. Recycling of platelet phosphorylation and cytoskeletal assembly. J Cell Biol. 1984 Jan;98(1):8–15. doi: 10.1083/jcb.98.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J. L., Molish I. R., Rigmaiden M., Stewart G. Evidence for a role of myosin phosphorylation in the initiation of the platelet shape change response. J Biol Chem. 1984 Aug 10;259(15):9826–9831. [PubMed] [Google Scholar]
- Funder J., Hershco L., Rothstein A., Livne A. Na+/H+ exchange and aggregation of human platelets activated by ADP: the exchange is not required for aggregation. Biochim Biophys Acta. 1988 Mar 3;938(3):425–433. doi: 10.1016/0005-2736(88)90140-x. [DOI] [PubMed] [Google Scholar]
- Gorman D. J., Castaldi P. A., Zola H., Berndt M. C. Preliminary functional characterization of a 24,000 dalton platelet surface protein involved in platelet activation. Nouv Rev Fr Hematol. 1985;27(4):255–259. [PubMed] [Google Scholar]
- Griendling K. K., Berk B. C., Alexander R. W. Evidence that Na+/H+ exchange regulates angiotensin II-stimulated diacylglycerol accumulation in vascular smooth muscle cells. J Biol Chem. 1988 Aug 5;263(22):10620–10624. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Daniel J. L., Kendrick-Jones J., Rink T. J. Relationship between cytoplasmic free calcium and myosin light chain phosphorylation in intact platelets. Biochem J. 1985 Dec 1;232(2):373–377. doi: 10.1042/bj2320373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hato T., Ikeda K., Yasukawa M., Watanabe A., Kobayashi Y. Exposure of platelet fibrinogen receptors by a monoclonal antibody to CD9 antigen. Blood. 1988 Jul;72(1):224–229. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Higashihara M., Maeda H., Yatomi Y., Takahata K., Oka H., Kume S. The platelet protein phosphorylation induced by a monoclonal antibody against human platelets (TP82). Biochem Biophys Res Commun. 1985 Nov 27;133(1):306–313. doi: 10.1016/0006-291x(85)91876-5. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Kawamoto S., Hidaka H. Serotonin secretion from human platelets may be modified by Ca2+-activated, phospholipid-dependent myosin phosphorylation. J Biol Chem. 1984 Dec 10;259(23):14321–14323. [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Modderman P. W., Huisman H. G., van Mourik J. A., von dem Borne A. E. A monoclonal antibody to the human platelet glycoprotein IIb/IIIa complex induces platelet activation. Thromb Haemost. 1988 Aug 30;60(1):68–74. [PubMed] [Google Scholar]
- Morel M. C., Lecompte T., Champeix P., Favier R., Potevin F., Samama M., Salmon C., Kaplan C. PL2-49, a monoclonal antibody against glycoprotein IIb which is a platelet activator. Br J Haematol. 1989 Jan;71(1):57–63. doi: 10.1111/j.1365-2141.1989.tb06275.x. [DOI] [PubMed] [Google Scholar]
- Newman R. A., Sutherland D. R., Lebien T. W., Kersey J. H., Greaves M. F. Biochemical characterisation of leukaemia-associated antigen p24 defined by the monoclonal antibody BA-2. Biochim Biophys Acta. 1982 Mar 4;701(3):318–327. doi: 10.1016/0167-4838(82)90234-5. [DOI] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Pollard H. B., Tack-Goldman K., Pazoles C. J., Creutz C. E., Shulman N. R. Evidence for control of serotonin secretion from human platelets by hydroxyl ion transport and osmotic lysis. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5295–5299. doi: 10.1073/pnas.74.12.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock W. K., Rink T. J., Irvine R. F. Liberation of [3H]arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochem J. 1986 May 1;235(3):869–877. doi: 10.1042/bj2350869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendu F., Boucheix C., Lebret M., Bourdeau N., Benoit P., Maclouf J., Soria C., Levy-Toledano S. Mechanisms of the mAb ALB6(CD9) induced human platelet activation: comparison with thrombin. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1397–1404. doi: 10.1016/0006-291x(87)90805-9. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E. Activation of human platelet phospholipase C by ionophore A23187 is totally dependent upon cyclo-oxygenase products and ADP. Biochem J. 1984 Aug 15;222(1):103–110. doi: 10.1042/bj2220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld S. I., Looney R. J., Leddy J. P., Phipps D. C., Abraham G. N., Anderson C. L. Human platelet Fc receptor for immunoglobulin G. Identification as a 40,000-molecular-weight membrane protein shared by monocytes. J Clin Invest. 1985 Dec;76(6):2317–2322. doi: 10.1172/JCI112242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicki J. P., Venton D. L., Le Breton G. C. The thromboxane antagonist, 13-azaprostanoic acid, inhibits arachidonic acid-induced Ca2+ release from isolated platelet membrane vesicles. Biochim Biophys Acta. 1983 Mar 22;751(1):66–73. doi: 10.1016/0005-2760(83)90257-6. [DOI] [PubMed] [Google Scholar]
- Sano K., Takai Y., Yamanishi J., Nishizuka Y. A role of calcium-activated phospholipid-dependent protein kinase in human platelet activation. Comparison of thrombin and collagen actions. J Biol Chem. 1983 Feb 10;258(3):2010–2013. [PubMed] [Google Scholar]
- Santoro S. A., Rajpara S. M., Staatz W. D., Woods V. L., Jr Isolation and characterization of a platelet surface collagen binding complex related to VLA-2. Biochem Biophys Res Commun. 1988 May 31;153(1):217–223. doi: 10.1016/s0006-291x(88)81211-7. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Brass L. F. Induction of the fibrinogen receptor on human platelets by intracellular mediators. J Biol Chem. 1987 Jan 25;262(3):992–1000. [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Ca2+ mobilization primes protein kinase C in human platelets. Ca2+ and phorbol esters stimulate platelet aggregation and secretion synergistically through protein kinase C. Biochem J. 1988 Oct 1;255(1):309–318. [PMC free article] [PubMed] [Google Scholar]
- Siffert W., Akkerman J. W. Protein kinase C enhances Ca2+ mobilization in human platelets by activating Na+/H+ exchange. J Biol Chem. 1988 Mar 25;263(9):4223–4227. [PubMed] [Google Scholar]
- Tandon N. N., Kralisz U., Jamieson G. A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989 May 5;264(13):7576–7583. [PubMed] [Google Scholar]
- Tohmatsu T., Hattori H., Nagao S., Ohki K., Nozawa Y. Reversal by protein kinase C inhibitor of suppressive actions of phorbol-12-myristate-13-acetate on polyphosphoinositide metabolism and cytosolic Ca2+ mobilization in thrombin-stimulated human platelets. Biochem Biophys Res Commun. 1986 Jan 29;134(2):868–875. doi: 10.1016/s0006-291x(86)80500-9. [DOI] [PubMed] [Google Scholar]
- Zavoico G. B., Cragoe E. J., Jr Ca2+ mobilization can occur independent of acceleration of Na+/H+ exchange in thrombin-stimulated human platelets. J Biol Chem. 1988 Jul 15;263(20):9635–9639. [PubMed] [Google Scholar]


