Abstract
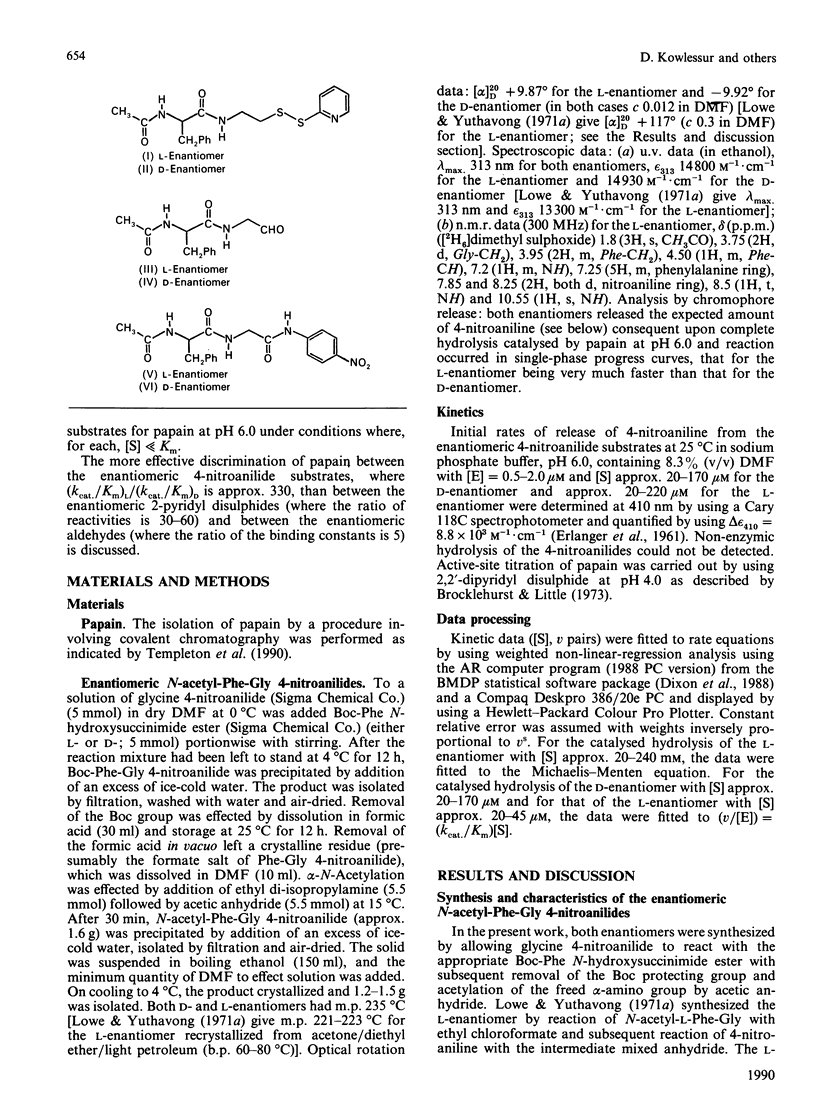
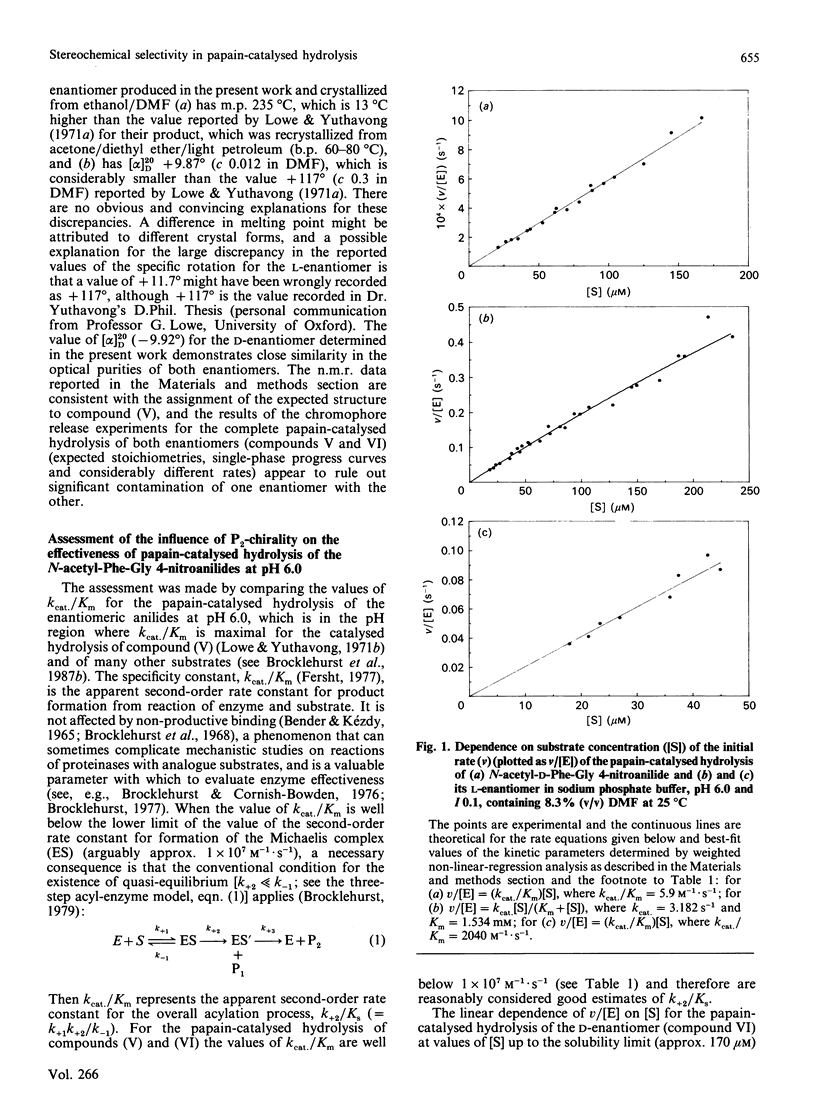
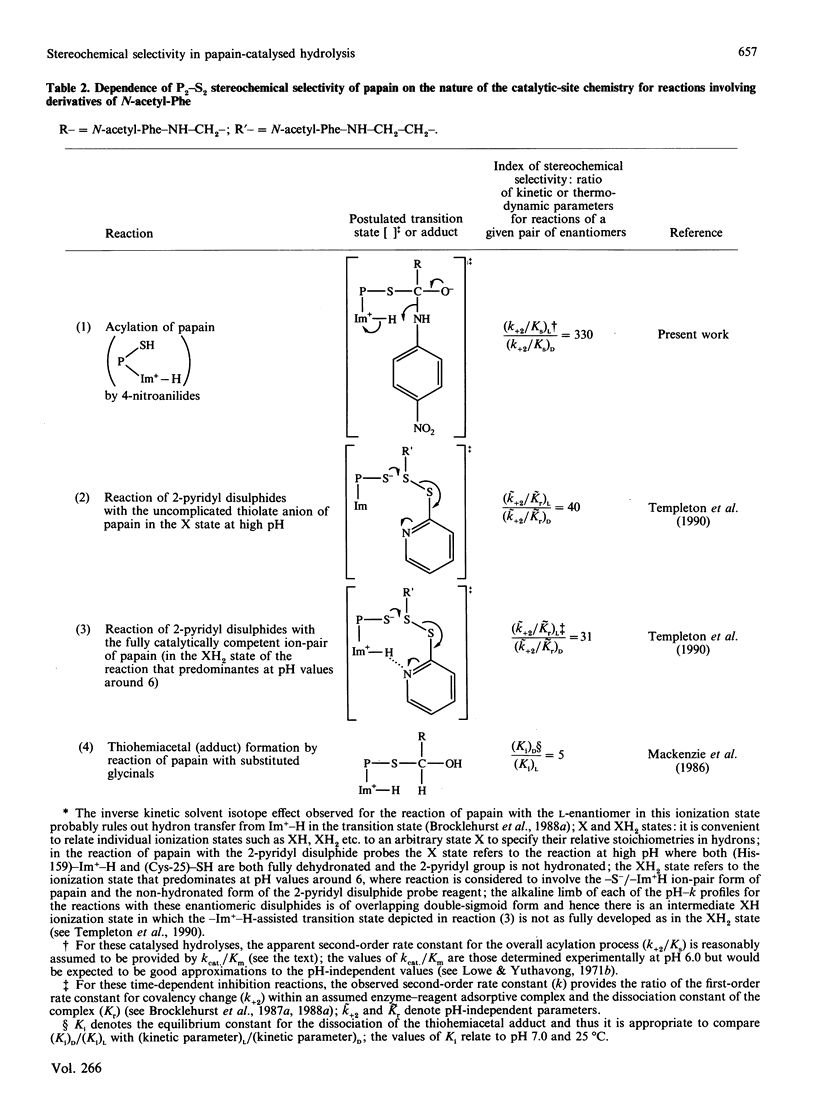
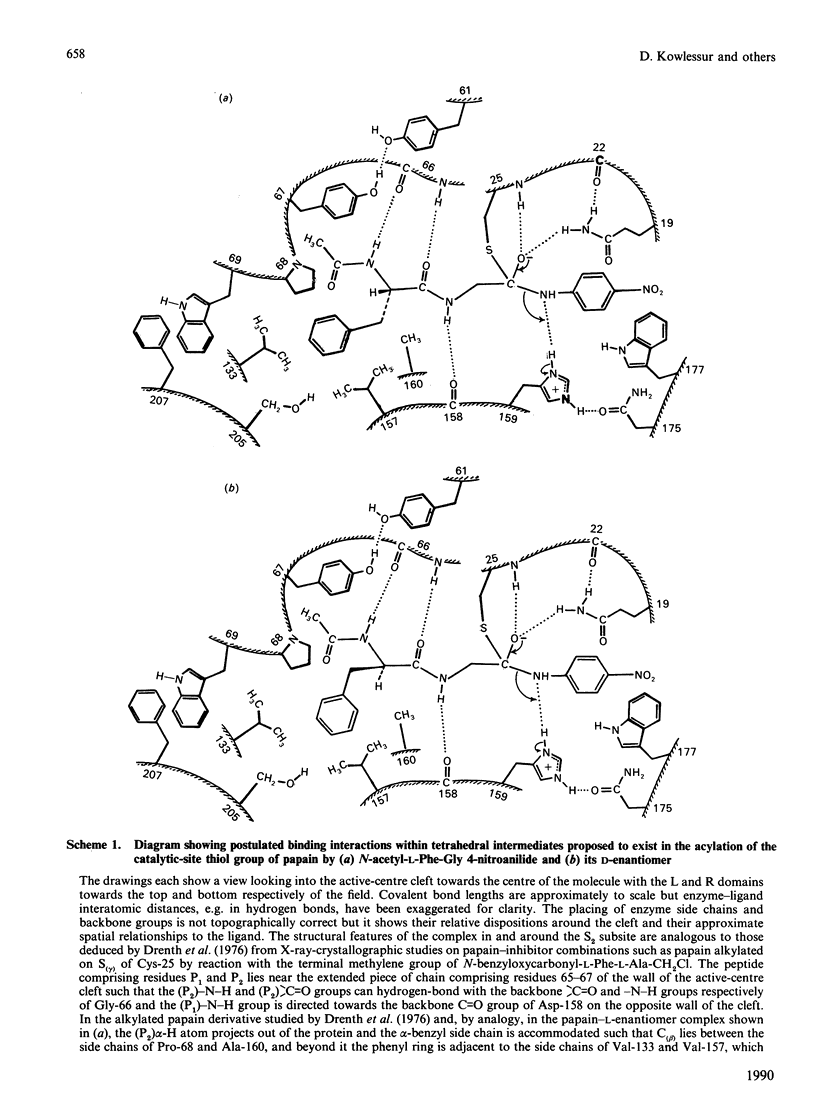
1. N-Acetyl-L-phenylalanylglycine 4-nitroanilide and its D-enantiomer were synthesized and characterized and used as substrates with which to evaluate stereochemical selectivity in papain (EC 3.4.22.2)-catalysed hydrolysis. 2. Kinetic analysis at pH 6.0, I 0.1, 8.3% (v/v) NN-dimethylformamide and 25 degrees C by using initial-rate data with [S] much less than Km and weighted non-linear regression provided values of kcat./Km for the catalysed hydrolysis of both enantiomers as (kcat./Km)L = 2040 +/- 48 M-1.S-1 and (kcat./Km)D = 5.9 +/- 0.07 M-1.S-1. These data, taken together with individual values of kcat. and Km for the hydrolysis of the L-enantiomer (a) estimated in the present work as kcat. = 3.2 +/- 1.2 S-1 and Km = 1.5 +/- 0.6 mM and (b) reported by Lowe & Yuthavong [(1971) Biochem. J. 124, 107-115] for the reaction at pH 6.0 in 10% (v/v) NN-dimethylformamide and 35 degrees C, as kcat. = 1.3 +/- 0.2 S-1 and Km = 0.88 +/- 0.1 mM, suggest that (kcat./Km)L congruent to 2000 M-1.S-1 and thus that (kcat./Km)L/(kcat./Km)D congruent to 330.3. Model building indicates that both enantiomeric 4-nitroanilides can bind to papain such that the phenyl ring of the N-acetylphenylalanyl group makes hydrophobic contacts in the S2 subsite with preservation of mechanistically relevant hydrogen-bonding interactions and that the main difference is in the positioning of the beta-methylene group. 4. The dependence of P2-S2 stereochemical selectivity of papain on the nature of the catalytic-site chemistry for reactions involving derivatives of N-acetylphenylalanine is discussed. The variation in the index of stereochemical selectivity (ratio of the appropriate kinetic or thermodynamic parameter for a given pair of enantiomeric ligands), from 330 for the overall acylation process of the catalytic act, through 40 and 31 for the reaction at electrophilic sulphur in 2-pyridyl disulphides respectively without and with assistance by (His-159)-Im(+)-H, to 5 for the formation of thiohemiacetal adducts by reaction at aldehydic carbon, is interpreted in terms of the extent to which conformational variation of the bound ligand in the catalytic-site region permits the binding mode of the -CH2-Ph group of the D-enantiomer to approach that of the L-enantiomer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbóth B., Stokum E., Khan I. U., Polgár L. Mechanism of action of cysteine proteinases: oxyanion binding site is not essential in the hydrolysis of specific substrates. Biochemistry. 1985 Jan 29;24(3):606–609. doi: 10.1021/bi00324a010. [DOI] [PubMed] [Google Scholar]
- BENDER M. L., KEZDY J. MECHANISM OF ACTION OF PROTEOLYTIC ENZYMES. Annu Rev Biochem. 1965;34:49–76. doi: 10.1146/annurev.bi.34.070165.000405. [DOI] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Brocklehurst S. M., Kowlessur D., O'Driscoll M., Patel G., Salih E., Templeton W., Thomas E., Topham C. M., Willenbrock F. Supracrystallographic resolution of interactions contributing to enzyme catalysis by use of natural structural variants and reactivity-probe kinetics. Biochem J. 1988 Dec 1;256(2):543–558. doi: 10.1042/bj2560543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Cornish-Bowden A. The pre-eminence of k(cat) in the manifestation of optimal enzymic activity delineated by using the Briggs-Haldane two-step irreversible kinetic model. Biochem J. 1976 Oct 1;159(1):165–166. doi: 10.1042/bj1590165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Crook E. M., Wharton C. W. The kinetic analysis of hydrolytic enzyme catalyses: Consequences of non-productive binding. FEBS Lett. 1968 Nov;2(1):69–73. doi: 10.1016/0014-5793(68)80103-6. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K. Evolution of enzyme catalytic power. Characteristics of optimal catalysis evaluated for the simplest plausible kinetic model. Biochem J. 1977 Apr 1;163(1):111–116. doi: 10.1042/bj1630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Kowlessur D., O'Driscoll M., Patel G., Quenby S., Salih E., Templeton W., Thomas E. W., Willenbrock F. Substrate-derived two-protonic-state electrophiles as sensitive kinetic specificity probes for cysteine proteinases. Activation of 2-pyridyl disulphides by hydrogen-bonding. Biochem J. 1987 May 15;244(1):173–181. doi: 10.1042/bj2440173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Kowlessur D., Patel G., Templeton W., Quigley K., Thomas E. W., Wharton C. W., Willenbrock F., Szawelski R. J. Consequences of molecular recognition in the S1-S2 intersubsite region of papain for catalytic-site chemistry. Change in pH-dependence characteristics and generation of an inverse solvent kinetic isotope effect by introduction of a P1-P2 amide bond into a two-protonic-state reactivity probe. Biochem J. 1988 Mar 15;250(3):761–772. doi: 10.1042/bj2500761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. The equilibrium assumption is valid for the kinetic treatment of most time-dependent protein-modification reactions. Biochem J. 1979 Sep 1;181(3):775–778. doi: 10.1042/bj1810775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J., Kalk K. H., Swen H. M. Binding of chloromethyl ketone substrate analogues to crystalline papain. Biochemistry. 1976 Aug 24;15(17):3731–3738. doi: 10.1021/bi00662a014. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Kowlessur D., Topham C. M., Thomas E. W., O'Driscoll M., Templeton W., Brocklehurst K. Identification of signalling and non-signalling binding contributions to enzyme reactivity. Alternative combinations of binding interactions provide for change in transition-state geometry in reactions of papain. Biochem J. 1989 Mar 15;258(3):755–764. doi: 10.1042/bj2580755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G., Yuthavong Y. Kinetic specificity in papain-catalysed hydrolyses. Biochem J. 1971 Aug;124(1):107–115. doi: 10.1042/bj1240107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G., Yuthavong Y. pH-dependence and structure-activity relationships in the papain-catalysed hydrolysis of anilides. Biochem J. 1971 Aug;124(1):117–122. doi: 10.1042/bj1240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie N. E., Grant S. K., Scott A. I., Malthouse J. P. 13C NMR study of the stereospecificity of the thiohemiacetals formed on inhibition of papain by specific enantiomeric aldehydes. Biochemistry. 1986 Apr 22;25(8):2293–2298. doi: 10.1021/bi00356a066. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Angus R. H., Carey P. R. Comparison of the kinetics of the papain-catalyzed hydrolysis of glycine- and alanine-based esters and thiono esters. Biochemistry. 1988 Jan 12;27(1):264–268. doi: 10.1021/bi00401a040. [DOI] [PubMed] [Google Scholar]
- Templeton W., Kowlessur D., Thomas E. W., Topham C. M., Brocklehurst K. A re-appraisal of the structural basis of stereochemical recognition in papain. Insensitivity of binding-site-catalytic-site signalling to P2-chirality in a time-dependent inhibition. Biochem J. 1990 Mar 15;266(3):645–651. doi: 10.1042/bj2660645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


