Abstract
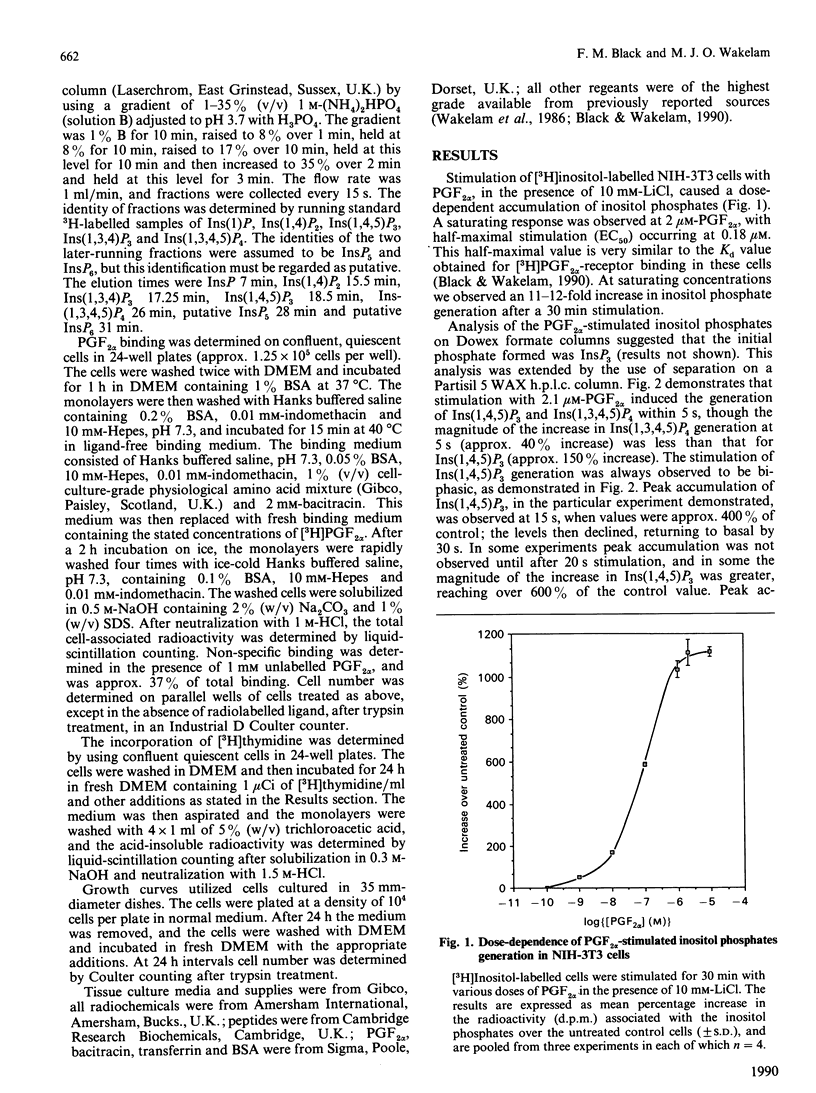
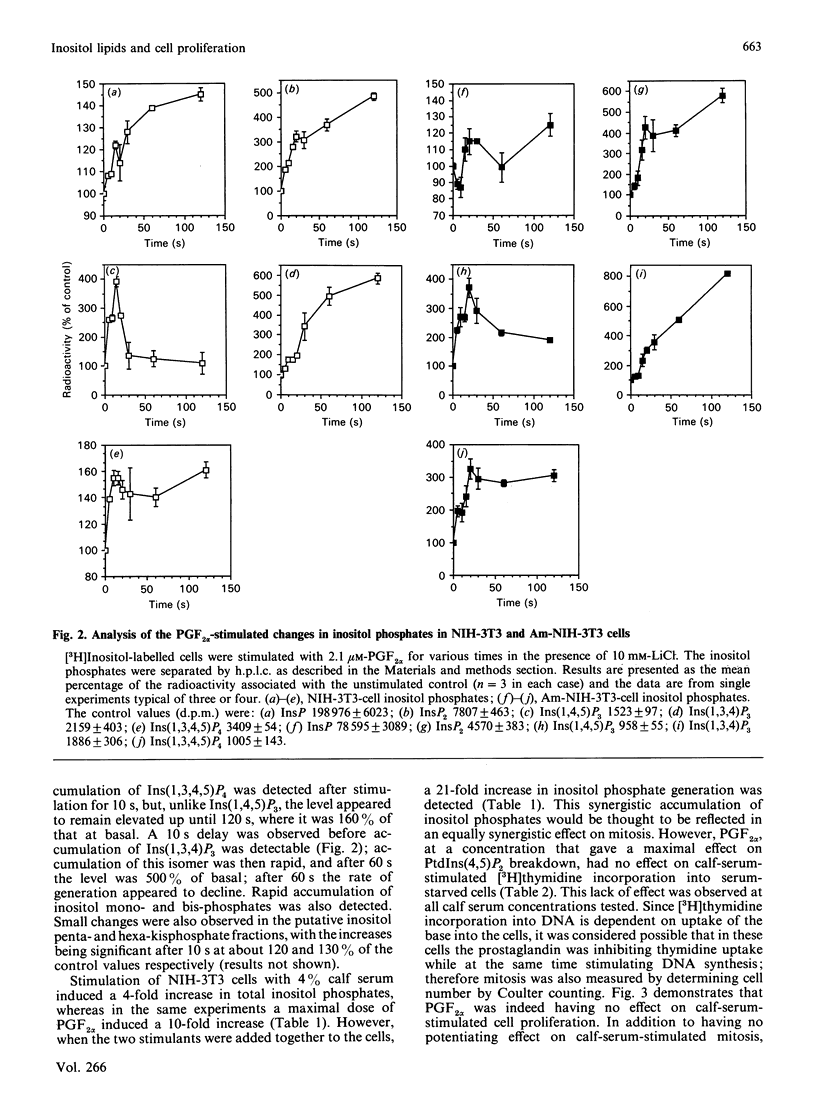
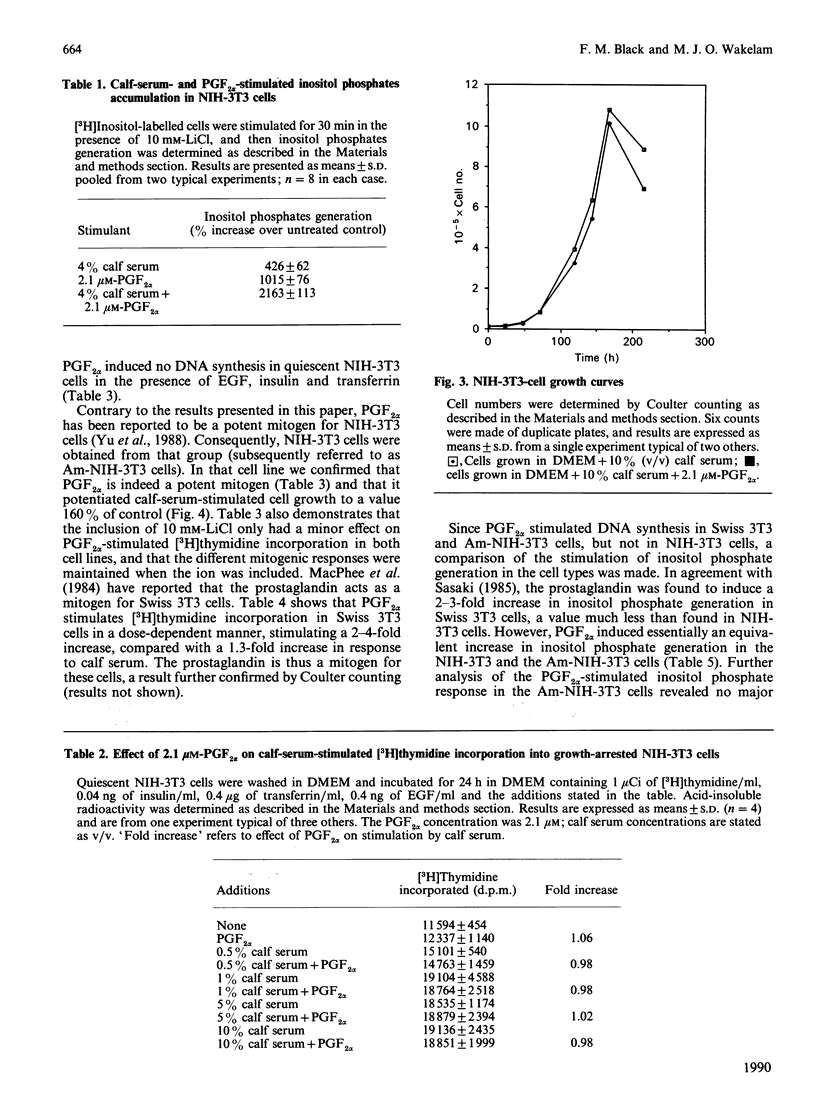
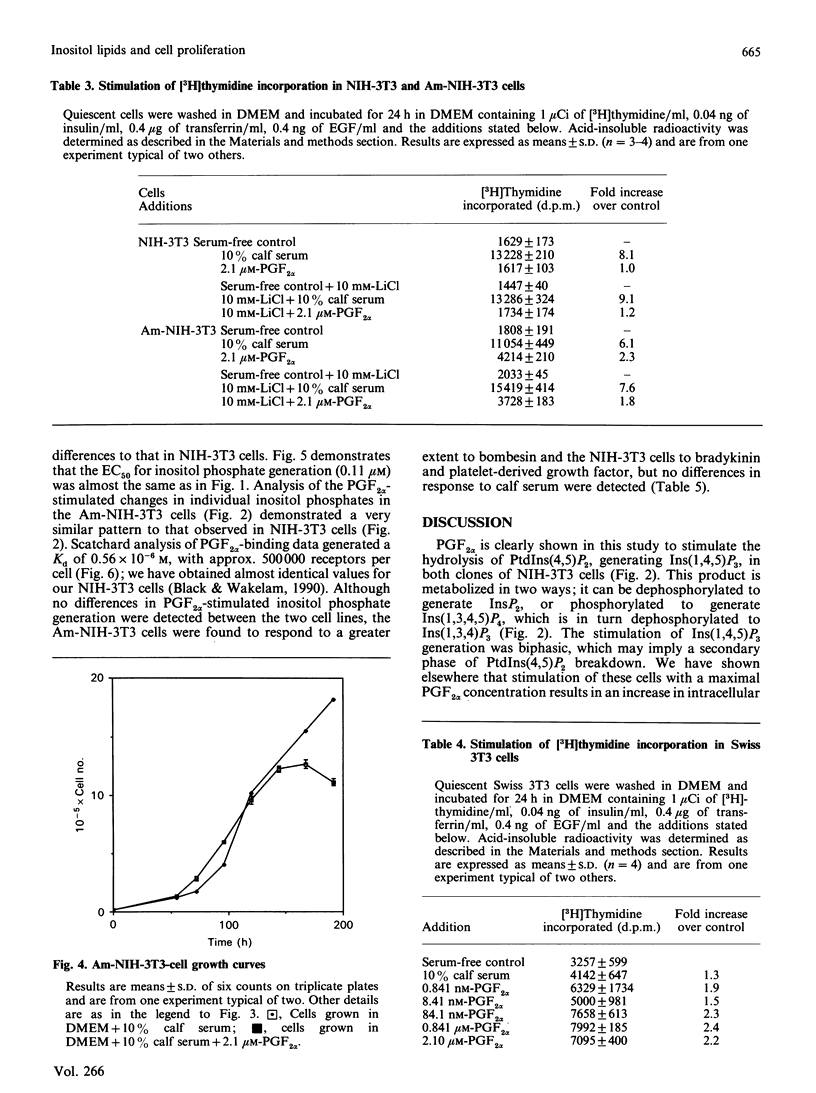
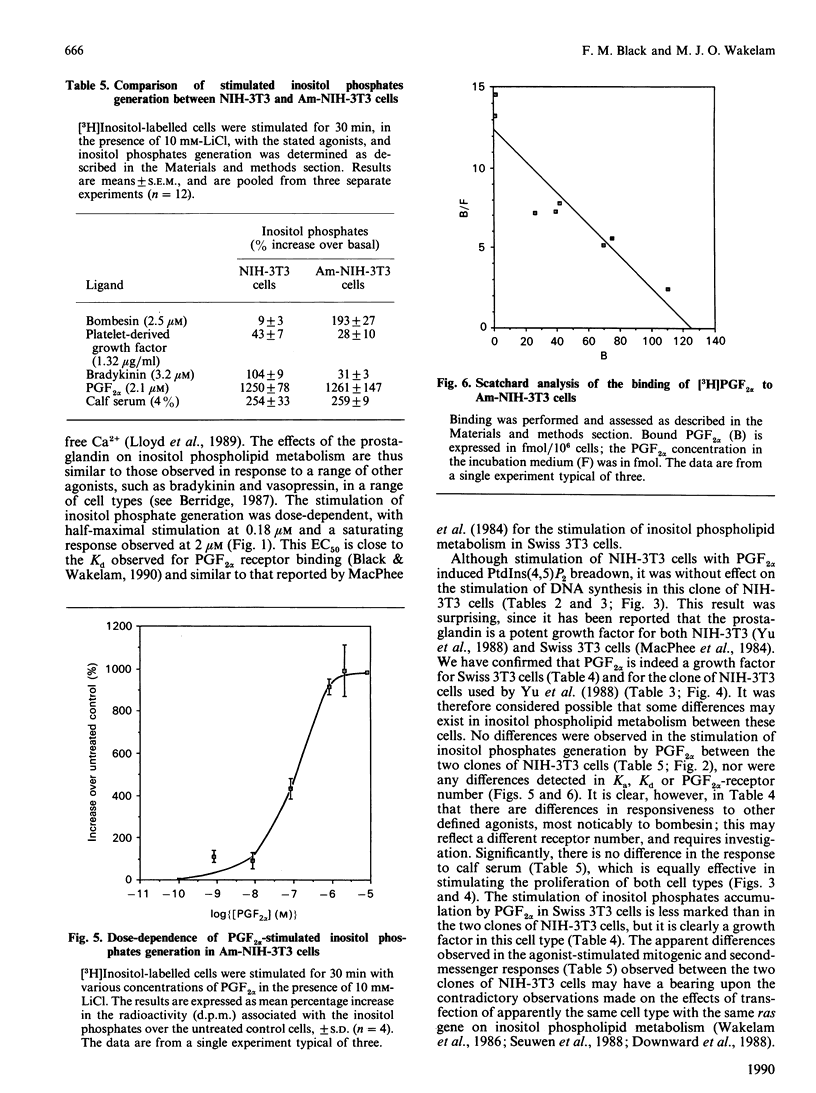
Stimulation of NIH-3T3 cells with prostaglandin F2 alpha (PGF2 alpha) caused a dose- and time-dependent generation of inositol phosphates. The first detectable changes were in the levels of Ins(1,4,5)P3 and Ins(1,3,4,5)P4. Increases in Ins(1,3,4)P3, InsP2 and InsP were detected later, and only minor changes were observed in putative InsP5 or InsP6. The accumulation of inositol phosphates was synergistically increased by the addition of calf serum, whereas PGF2 alpha had no effects on cell proliferation in either the presence or the absence of calf serum. Stimulation of a different clone of NIH-3T3 cells (AmNIH-3T3) or Swiss 3T3 cells with PGF2 alpha resulted in both inositol phospholipid breakdown and cell proliferation. No differences were found in the characteristics of PGF2 alpha-stimulated inositol phosphate generation between the two clones of NIH-3T3 cells, nor was there any difference in receptor number of Kd. These results question the role of inositol phospholipid breakdown in mitogenesis and demonstrate significant differences in the biochemical properties of apparently the 'same' cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol lipids and cell proliferation. Biochim Biophys Acta. 1987 Apr 20;907(1):33–45. doi: 10.1016/0304-419x(87)90017-5. [DOI] [PubMed] [Google Scholar]
- Downward J., de Gunzburg J., Riehl R., Weinberg R. A. p21ras-induced responsiveness of phosphatidylinositol turnover to bradykinin is a receptor number effect. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5774–5778. doi: 10.1073/pnas.85.16.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Lloyd A. C., Davies S. A., Crossley I., Whitaker M., Houslay M. D., Hall A., Marshall C. J., Wakelam M. J. Bombesin stimulation of inositol 1,4,5-trisphosphate generation and intracellular calcium release is amplified in a cell line overexpressing the N-ras proto-oncogene. Biochem J. 1989 Jun 15;260(3):813–819. doi: 10.1042/bj2600813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphee C. H., Drummond A. H., Otto A. M., Jimenez de Asua L. Prostaglandin F2 alpha stimulates phosphatidylinositol turnover and increases the cellular content of 1,2-diacylglycerol in confluent resting Swiss 3T3 cells. J Cell Physiol. 1984 Apr;119(1):35–40. doi: 10.1002/jcp.1041190107. [DOI] [PubMed] [Google Scholar]
- Sasaki T. Formation of inositol phosphates and calcium mobilization in Swiss 3T3 cells in response to prostaglandin F2 alpha. Prostaglandins Leukot Med. 1985 Aug;19(2):153–159. doi: 10.1016/0262-1746(85)90081-2. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rozengurt E. Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7778–7782. doi: 10.1073/pnas.79.24.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuwen K., Lagarde A., Pouysségur J. Deregulation of hamster fibroblast proliferation by mutated ras oncogenes is not mediated by constitutive activation of phosphoinositide-specific phospholipase C. EMBO J. 1988 Jan;7(1):161–168. doi: 10.1002/j.1460-2075.1988.tb02796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Blakeley D. M., Corps A. N., Berridge M. J., Brown K. D. Effects of pertussis toxin on growth factor-stimulated inositol phosphate formation and DNA synthesis in Swiss 3T3 cells. Biochem J. 1988 Feb 1;249(3):917–920. doi: 10.1042/bj2490917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tones M. A., Sharif N. A., Hawthorne J. N. Phospholipid turnover during cell-cycle traverse in synchronous Chinese-hamster ovary cells. Mitogenesis without phosphoinositide breakdown. Biochem J. 1988 Jan 1;249(1):51–56. doi: 10.1042/bj2490051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]


