Abstract
Background
Methamphetamine is an emerging drug threat. The disparity in cardiomyopathy-associated hospital admissions among methamphetamine users (CAHMA) over the decade remains unknown.
Objectives
The purpose of this study was to determine the trends and prevalence of CAHMA by age, sex, race, and geographical region.
Methods
We used data from 2008 to 2020 from the National Inpatient Sample database. We identified 12,845,919 cardiomyopathy-associated hospital admissions; among them, 222,727 were diagnosed as methamphetamine users. A generalized linear model with binomial link function was used to compute the prevalence ratio and 95% CI. Those who used other substances along with methamphetamine were excluded from the analysis.
Results
CAHMA increased by 231% (P trend <0.001) from 2008 to 2020. CAHMA increased 345% for men (P trend <0.001) and 122% for women (P trend <0.001), 271% for non-Hispanic White (P trend <0.001), 254% for non-Hispanic Black (p trend <0.001), 565% for Hispanic (P trend <0.001), and 645% for non-Hispanic Asian (P trend <0.001) population. CAHMA also increased significantly in the West region (530%) (P trend <0.001) and South region (200%) (P trend <0.001) of the United States. Men, Hispanic population, age groups 26 to 40 and 41 to 64 years, and Western regions showed a significantly higher uptrend than their counterparts (P trend <0.001).
Conclusions
CAHMA have increased significantly in the United States. Men, Hispanics, non-Hispanic Asian, age groups 41 to 64. and western regions showed a higher proportional increase highlighting gender-based, racial/ethnic, and regional disparities over the study period.
Key words: cardiomyopathy, disparity, hospital admission, methamphetamine, trend
Central Illustration
The abuse of psychostimulant drugs, such as methamphetamine, is a burgeoning global public health concern, with the estimated number of annual users surpassing 27 million individuals worldwide in 2017.1 Of all these substances, methamphetamine, also known as "Meth," carries one of the highest burdens of disease associated with drug use in the United States.2 Methamphetamine use has been associated with a wide range of cardiovascular diseases (CVDs), including cardiomyopathy, pulmonary hypertension, and coronary artery disease.3 Interestingly, a growing body of evidence in the literature shows that long-term methamphetamine use may also be associated with structural and functional changes in the heart, such as cardiac fibrosis, systolic dysfunction, and a predisposition to arrhythmias.
Methamphetamine use-associated hospital admissions (MAH) have increased over time, and the effects of methamphetamine have been shown to vary based on various demographic factors.4 For example, studies have shown that the risk of heart failure and CVD in methamphetamine users is higher in the younger population compared to non-methamphetamine users (median age 49 years).3,5, 6, 7 Additionally, studies have revealed discrepancies among CVD in methamphetamine users, with non-Hispanic black, Hispanic, and non-Hispanic Asian/Pacific Islander individuals exhibiting a higher risk of CVD.5, 6, 7, 8 In addition, geographical studies have revealed that the use of methamphetamine is more prevalent in certain regions of the United States, such as the West and Southwest, and that individuals in these regions have a higher risk of developing a CVD related to methamphetamine use than in other regions.5 Finally, studies have shown increased hospitalizations for methamphetamine-associated heart failure.5 Since methamphetamine is known to cause cardiomyopathy, a primary cause of heart failure, we sought to investigate the prevalence of the trend of cardiomyopathy among methamphetamine users in the United States over the last decade and investigate how this was affected by age, race/ethnicity, sex, and geographical regions.
Methods
The National Inpatient Database (NIS) is a comprehensive deidentified database developed by the Healthcare Cost and Utilization Project (HCUP). The NIS is one of the country's largest and most widely used health care databases, with over 7 million hospital admissions each year. The NIS covers roughly 97% of Americans since more states have recently joined HCUP. The data were collected from hospitals in all 50 states. The NIS data is publicly available and can be accessed through the HCUP website. We identified all adults (>18 years of age) hospitalized with a diagnosis of cardiomyopathy who were also methamphetamine users. Only methamphetamine users were included; multiple drug users (those who used other drugs with methamphetamine) were excluded. Overall, cardiomyopathy patients were selected using the International Classification of Diseases (ICD)-9 = 425, ICD-10 = I42, and I43, and methamphetamine-user patients were selected using ICD-9 = 304.x, 305.x, ICD-10 = F15.1x, and F15.2x codes.5 NIS data uses a standardized diagnosis coding and classification system that provides the opportunity to produce a consistent and reproducible trend analysis for different disease-related hospital utilization at different time points. Previous studies showed that this coding correlates with clinical evidence of methamphetamine abuse, especially in heart failure patients9, 10, 11, 12 A complete list of the ICD-9 and ICD-10 codes for cardiomyopathy-associated hospital admission (CAH), methamphetamine users, and states in each region are presented in Supplemental Tables 1 to 3, respectively. The NIS data are nonidentifiable and, therefore, institutional review board-exempt. We followed COREQ reporting guidelines.
Statistical analysis
To generate national estimates for trend analysis, trend weight was used for years before 2012, and regular discharge weight was used for years after 2012. All hospital discharges in a specific NIS year have the same discharge weight. Therefore, the trend weight files were integrated with the original NIS files by year and hospital ID. Continuous variables were totaled for unweighted and national representative data, and categorical variables were summarized as frequencies and percentages. To account for NIS sample design and sample weight, design-based t-tests were used for continuous values, and design-based chi-squared tests were used for categorical values.13 The prevalence ratio (PR) of cardiomyopathy-associated hospital admission among methamphetamine users (CAHMA) was calculated as the number of CAHMA cases among methamphetamine users divided by the number of CAHMA cases among non-methamphetamine users. The frequency of missing values was summarized, and Little’s MCAR (missing completely at random) was used to screen for missing data patterns. A nonsignificant P value (P > 0.05) represented randomly missing. A generalized linear model with binomial link function was used to compute the PR and 95% CI.14, 15, 16 The model was adjusted for gender, age, race, primary payer, median household income, length of hospital stay, U.S. region, anemia, arthritis, chronic pulmonary disease, congestive heart failure, coagulopathy, depression, diabetes, liver disease, hypertension, obesity, peripheral vascular disorders, pulmonary circulation disorders, and renal failure. We used linear trend analysis to calculate the P value and the Cochran-Armitage trend test to compare the trend stratified by age, sex, race, and region. The design and analytic guidelines for NIS data have been described before.17,18 Analysis was performed following the HCUP guidelines.19,20 All statistical analysis was performed in R, version 4.2.2. A 2-sided P value <0.05 was considered significant.
Results
For the period from 2008 to 2020, there were 395,772,653 national representative hospital admissions (mean hospital admission of 30,444,051 per year). There were 1,268,584 MAH admissions and 12,845,919 CAHs. Among them, 222,727 cardiomyopathy patients were methamphetamine users.
Demographic characteristics of cardiomyopathy-associated hospital admissions patients
Overall, cardiomyopathy patients were disproportionately men (59.25%) compared to women (40.75%). In addition, most CAH patients were 41 years of age or older (94%), with only 6% <41 years old. By race, the 2 most prominent hospital admissions were for non-Hispanic White (63.41%) and non-Hispanic black (24.04%) patients. For insurance, the predominant payer was Medicare (64.05%). The demographic characteristics of the cardiomyopathy patients are shown in Supplemental Table 5.
Temporal trend of cardiomyopathy-associated hospital admissions patients
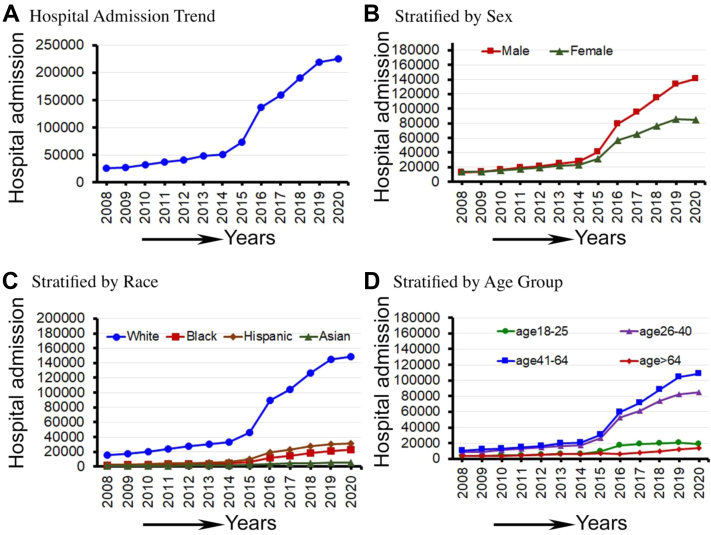
From 2008 to 2020, there were 12,845,920 national representative CAH (mean admission of 988,148 per year); the number increased significantly (12%) during this time (P for trend <0.001). In addition, CAH was significantly increased in men (18%) compared to women (2%) (P < 0.001), non-Hispanic Black (47%), non-Hispanic White (30%), Hispanics (51%), and non-Hispanic Asians (48%) compared to other races, and in patients in the 41- to 64-year-old age groups (18.84%) compared to patients in other age groups (P < 0.001). The trend of CAH from 2008 to 2020 is shown in Figure 1.
Figure 1.
Cardiomyopathy-Associated Hospital Admissions From 2028 to 2020 in the U.S.
(A) The trend of cardiomyopathy-associated hospital admissions and hospital admissions stratified by (B) sex, (C) race, and (D) age groups from 2008 to 2020 in the United States.
Demographic characteristics of methamphetamine users hospital admissions patients
Between 2008 and 2020, national representative MAH increased from 2.05% to 17.83%. Methamphetamine use was higher among men, who comprised approximately 60% of methamphetamine cases vs 40% for women. The mean age for methamphetamine users were 42, with over 80% being between the ages of 26 and 64. The highest proportion of methamphetamine users was non-Hispanic White (68.84%), followed by 14.2% Hispanic and 9.78% non-Hispanic Black. The primary payment method for hospitalization for methamphetamine users was Medicaid (48.51%). The demographic characteristics of MAH are shown in Supplemental Table 4.
Temporal trend of methamphetamine-associated hospital admission patients
From 2008 to 2020, MAH increased significantly by 769% (p-trend <0.001). Between 2008 and 2020, MAH increased significantly for both men (1,021%) and women (534%) (P for trend <0.001). From 2008 to 2020, hospital admissions were significantly higher for men than women (P < 0.001). Hospital admission by race for methamphetamine use also increased significantly (P for trend <0.001) and increased among Whites (878%), Blacks (1,170%), and Hispanics (1,291%) (P < 0.001). Among all age groups, hospital admission for methamphetamine use increased significantly by 485% for 18 to 25 years, 910% for 26 to 40 years, and 918% for the 41 to 64 age group (P for trend <0.001). All-cause mortality increased by 674% from 2008 to 2020 among MAH patients. The trend plots for MAH from 2008 to 2020 are shown in Figure 2.
Figure 2.
Methamphetamine Use Associated Hospital Admissions From 2008 to 2020 in the U.S.
(A) The trend of methamphetamine use-associated hospital admissions and hospital admissions stratified by (B) sex, (C) race, and (D) age groups from 2008 to 2020 in the United States.
Demographic characteristics of cardiomyopathy-associated hospital admissions among methamphetamine users patients
CAHMA was significantly higher in men compared to women (58.52% vs 41.48%, P < 0.0001). In comparing CAHMA vs nonusers, there were significantly more women in the methamphetamine users group compared to the non-methamphetamine users group (41.48% vs 40.74%, P < 0.0001). In addition, CAHMA was significantly higher in the 18 to 25 age group (2.44% vs 0.84%, P < 0.0001), 26 to 40 age group (15.61% vs 5.13%, P < 0.0001), and 41 to 64 age group (55.97% vs 36.37%, P < 0.0001). Broken by race/ethnicity, the CAHMA was prominent in non-Hispanic whites (62.92%) compared to non-Hispanic blacks (16.64%). Conversely, the Hispanic, non-Hispanic Asian or Pacific Islander, and non-Hispanic Native American groups made up a disproportionate amount of CAHMA (11.60%, 5.08%, 1.09%) compared to non-methamphetamine users (7.66%, 1.94%, 0.54%), respectively, with P < 0.0001. More detailed results for cardiomyopathy patients with and without methamphetamine use are shown in Table 1.
Table 1.
Demographic Characteristics of Cardiomyopathy Patients With and Without Methamphetamine Use From 2008-2020 in the United States
| Group | Cardiomyopathy Patients With Methamphetamine Use | Cardiomyopathy Patients Without Methamphetamine Use | P Value | |
|---|---|---|---|---|
| National estimates 2008-2020 | 222,727 | 12,623,193 | ||
| Sex | Men | 130,316 (58.52) | 7,480,077 (59.26) | <0.001 |
| Women | 92,362 (41.48) | 5,141,918 (40.74) | ||
| Age (y) | 18-25 | 5,446 (2.44) | 105,475 (0.84) | |
| 26-40 | 34,771 (15.61) | 647,868 (5.13) | ||
| 41-64 | 124,657 (55.97) | 4,591,269 (36.37) | ||
| ≥65 | 57,854 (25.98) | 7,278,582 (57.66) | ||
| Race/ethnicitya | White | 131,587 (62.92) | 7,499,521 (63.42) | <0.001 |
| Black | 34,790 (16.64) | 2,855,193 (24.14) | ||
| Hispanic | 24,252 (11.60) | 909,171 (7.69) | ||
| Asian or Pacific Islander | 10,628 (5.08) | 229,900 (1.94) | ||
| Native American | 2,277 (1.09) | 64,873 (0.55) | ||
| Other | 5,593 (2.67) | 267,256 (2.26) | ||
| Primary payer | Medicare | 80,406(36.17) | 8,134,607 (64.54) | <0.001 |
| Medicaid | 78,007 (35.09) | 1,493,770 (11.85) | ||
| Private insurance | 43,912 (19.75) | 2,209,832 (17.53) | ||
| Self-pay | 13,191 (5.93) | 451,667 (3.58) | ||
| No charge | 717 (0.32) | 45,137 (0.36) | ||
| Other | 6,056 (2.72) | 268,632 (2.13) | ||
| Median household Income ($) | <50,000 | 61,211 (29.13) | 4,004,804 (32.43) | <0.001 |
| 50,000-64,999 | 55,390 (26.36) | 3,212,653 (25.90) | ||
| 65,000-85,99 | 51,994 (24.74) | 2,864,399 (23.20) | ||
| >86,000 | 41,538 (19.77) | 2,294,405 (18.48) | ||
| Length of stay (d) | <3 d | 1,037,195 (40.34) | 5,098,918.41 (40.39) | <0.001 |
| 4-6 d | 738,610 (28.73) | 3,632,147.96 (28.77) | ||
| 7-9 d | 353,964 (13.77) | 1,740,210.64 (13.79) | ||
| 10-12 d | 167,402 (6.51) | 822,719.36 (6.52) | ||
| >12 d | 273,942 (10.65) | 1,328,752.52 (10.53) | ||
| U.S. region | Northeast | 24,655 (11.07) | 2,351,011 (18.602 | <0.001 |
| Midwest | 41,056 (18.43) | 2,928,078 (23.20) | ||
| South | 54,524 (24.48) | 5,230,268 (41.43) | ||
| West | 102,493 (46.02) | 2,113,837 (16.75) | ||
| Comorbidity | ||||
| Chronic pulmonary disease | Yes | 53,849 (25.71) | 3,480,212 (29.28) | <0.001 |
| Congestive heart failure | Yes | 68,070 (38.39) | 3,854,645 (35.47) | <0.001 |
| Coagulopathy | Yes | 14,411 (8.13) | 788,708 (7.26) | <0.001 |
| Depression | Yes | 23,947 (11.43) | 1,233,958 (10.38) | <0.001 |
| Diabetes | Yes | 54,837 (26.18) | 4,331,708 (36.44) | <0.001 |
| Liver disease | Yes | 9,429 (5.32) | 413,595 (3.81) | <0.001 |
| Obesity | Yes | 34,275 (16.36) | 2,114,986 (17.79) | 0.79 |
| Peripheral vascular disorders | Yes | 13,970 (6.67) | 1,323,774 (11.14) | <0.001 |
| Pulmonary circulation disorders | Yes | 10,068 (5.68) | 560,685 (5.16) | <0.001 |
Values are n (%).
Admitted patients were identified as non-Hispanic American Indian or Alaska Native, non-Hispanic Asian, non-Hispanic Black, Hispanic, and non-Hispanic White.
The geographical trend of cardiomyopathy-associated hospital admissions among methamphetamine users patients
Over time, MAH was higher in the western region (50.64%) (Table 1), and CAH was higher in the southern region (41.26%) (Table 2). As a result, CAHMA increased in the western region (46.02%). From 2008 to 2010, CAHMA remained the same across all the regions. However, in 2011, hospital admissions began to increase in the western region, followed in 2013 by a similar trend in the southern region. This upward trajectory persisted until 2018, when hospital admissions increased in the Midwest region. The geographical variation heatmap of CAHMA is shown in Figure 3.
Table 2.
Adjusted and Unadjusted Prevalence Ratio With 95% CI for Cardiomyopathy-Associated Hospital Admission Among Methamphetamine Users
| Group | Cardiomyopathy With Methamphetamine Use |
||||
|---|---|---|---|---|---|
| Unadjusted Prevalence Ratio | (95% CI) | Adjusted Prevalence Ratio | (95% CI) | ||
| Gender | Male | 1.00 (Reference) | 1.00 (Reference) | ||
| Female | 0.99 | (0.98-1.02) | 1.05 | (1.01-1.08) | |
| Age (y) | 18-25 | 1.00 (Reference) | 1.00 (Reference) | ||
| 26-40 | 1.93 | (1.81-2.07) | 2.12 | (1.96-2.3) | |
| 41-64 | 6.8 | (6.40-7.24) | 5.55 | (5.13-6.02) | |
| ≥65 | 45.26 | (42.32-48.45) | 32.84 | (29.89-36.12) | |
| Race/ethnicitya | White | 2.23 | (2.16-2.30) | 3.04 | (2.9-3.19) |
| Black | 0.87 | (0.84-0.90) | 1.35 | (1.29-1.42) | |
| Hispanic | 2.57 | (2.44-2.71) | 2.68 | (2.48-2.9) | |
| Asian or Pacific Islander | 0.6 | (0.55-0.66) | 0.82 | (0.71-0.94) | |
| Native American | 1.13 | (1.05-1.20) | 1.20 | (1.09-1.33) | |
| Other | 1.00 (Reference) | 1.00 (Reference) | |||
| Primary payer | Medicare | 1.00 (Reference) | 1.00 (Reference) | ||
| Medicaid | 5.26 | (5.14-5.37) | 0.87 | (0.84-0.91) | |
| Private insurance | 2.01 | (1.96-2.07) | 1.95 | (1.85-2.05) | |
| Self-pay | 2.95 | (2.83-3.08) | 0.64 | (0.6-0.68) | |
| No charge | 1.62 | (1.37-1.91) | 0.61 | (0.49-0.75) | |
| Other | 2.28 | (2.15-2.41) | 0.78 | (0.71-0.85) | |
| Median household Income ($) | <50,000 | 1.00 (Reference) | 1.00 (Reference) | ||
| 50,000-64,999 | 0.66 | (0.63-0.68) | 1.23 | (1.18-1.28) | |
| 65,000-85,99 | 0.51 | (0.49-0.52) | 1.43 | (1.37-1.49) | |
| >86,000 | 0.42 | (0.41-0.44) | 1.87 | (1.79-1.97) | |
| Length of hospital stay (d) | <3 d | 1.00 (Reference) | 1.00 (Reference) | ||
| 4-6 d | 1.25 | (1.22-1.28) | |||
| 7-9 d | 1.44 | (1.39-1.49) | |||
| 10-12 d | 1.58 | (1.51-1.65) | |||
| >12 d | 1.88 | (1.81-1.94) | |||
| U.S. region | Northeast | 1.00 (Reference) | 1.00 (Reference) | ||
| Midwest | 1.00 | (0.97-1.02) | 0.89 | (0.76-0.92) | |
| South | 1.21 | (1.17-1.24) | 1.02 | (0.92-1.89) | |
| West | 3.80 | (3.65-3.95) | 2.14 | (1.64-2.86) | |
| Death | Yes | 3.61 | (3.40-3.83) | 2.16 | (1.89-3.32) |
| Anemia | Yes | 2.23 | (2.15-2.31) | 1.47 | (1.4-1.55) |
| Arthritis | Yes | 2.41 | (2.23-2.61) | 1.37 | (1.23-1.53) |
| Chronic pulmonary disease | Yes | 1.63 | (1.59-1.67) | 0.97 | (0.93-1) |
| Congestive heart failure | Yes | 13.17 | (12.75-13.59) | 8.38 | (8.04-8.74) |
| Coagulopathy | Yes | 3.10 | (2.96-3.26) | 1.79 | (1.67-1.92) |
| Depression | Yes | 0.69 | (0.67-0.71) | 0.62 | (0.59-0.65) |
| Diabetes | Yes | 2.11 | (2.07-2.18) | 1.08 | (1.04-1.12) |
| Liver disease | Yes | 0.98 | (0.93-1.03) | 1.03 | (0.99-1.06) |
| Hypertension | Yes | 2.74 | (2.68-2.79) | 0.60 | (0.56-0.64) |
| Obesity | Yes | 1.91 | (1.85-1.97) | 1.53 | (1.47-1.61) |
| Peripheral vascular disorders | Yes | 3.64 | (3.46-3.83) | 1.38 | (1.28-1.49) |
| Pulmonary circulation disorders | Yes | 4.77 | (4.48-5.08) | 1.18 | (1.08-1.29) |
| Renal failure | Yes | 6.21 | (5.98-6.44) | 2.06 | (1.95-2.17) |
Admitted patients were identified as non-Hispanic American Indian or Alaska Native, non-Hispanic Asian, non-Hispanic Black, Hispanic, and non-Hispanic White.
Figure 3.
Geographical Variation of Cardiomyopathy-Associated Hospital Admissions Among Methamphetamine Users in the United States
Blue color indicated low admission regions, gray color indicated moderate admission regions and red color indicated high hospital admission regions.
Temporal trend of cardiomyopathy-associated hospital admissions with and without methamphetamine use patients
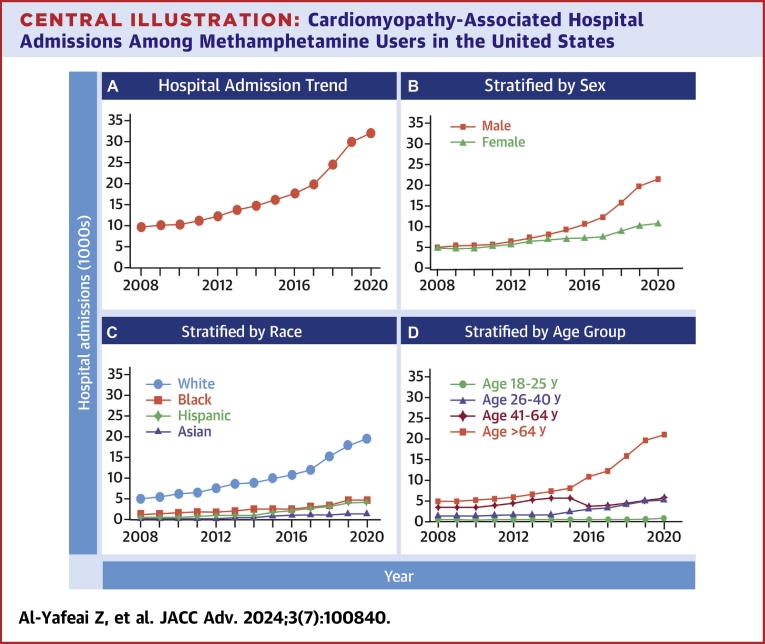
CAHMA increased by 231% (P for trend <0.001), and all-cause mortality increased by 195% (p trend <0.001) from 2008 to 2020. CAHMA increased 345% for men (P for trend <0.001) and 122% for women. (P for trend <0.001), 271% for non-Hispanic White (P for trend <0.001), 254% for non-Hispanic Black (P for trend <0.001), 565% for Hispanic (P for trend <0.001), and 645% for non-Hispanic Asian (P for trend <0.001) population. CAHMA also increased significantly in the West region (530%) (P for trend <0.001) and South region (200%) (P for trend <0.001) of the United States. The overall trend, stratified by sex, race, and age groups, for CAHMA is shown in Central Illustration.
Central Illustration.
Cardiomyopathy-Associated Hospital Admissions Among Methamphetamine Users in the United States
(A) The trend of cardiomyopathy-associated hospital admissions among methamphetamine users and hospital admissions stratified by (B) sex, (C) race, and (D) age groups from 2008 to 2020 in the United States.
Trend in prevalence of cardiomyopathy-associated hospital admission among methamphetamine users patients stratified by age, race, and sex
The PR of CAHMA was significantly higher for patients aged 41 to 64 years (6.80, 95% CI: 6.40-7.24) and >65 years (PR 45.26, 95% CI: 42.32-48.45) compared to patients aged 18 to 25 years. After adjusting for the sociodemographic and comorbidity variables, the adjusted prevalence ratio (aPR) became 32.84. Compared to men, the PR of CAHMA was lower for women (0.99, 95% CI: 0.98-1.02) and aPR (1.05, 95% CI: 1.01-1.08). The total number of CAHMA increased significantly for both men (∼4-fold) and women (∼2.5-fold) throughout the study period (P < 0.001).
The aPR of CAHMA was significantly higher in Hispanics (2.68, 95% CI: 2.48-2.90), non-Hispanic Whites (2.68, 95% CI: 2.48-2.90), and non-Hispanic Native Americans (1.20, 95% CI: 1.09-1.33) compared to that in non-Hispanic Blacks. Interestingly, the aPR of CAHMA was significantly lower for blacks compared to non-Hispanic whites (PR 1.35, 95% CI: 1.29-1.42). Non-Hispanic Native Americans, non-Hispanic Asians, and Hispanics showed the highest proportional increase from 2008 to 2020 (∼7-fold), (P < 0.0001); for non-Hispanic Asians (P < 0.000); and for non-Hispanic Native Americans (P < 0.0001).
The southern and western regions showed the highest PRs compared to the northeast; the aPR for the south was 1.02 (95% CI: 0.92-1.89), and the aPR for the west was 2.14 (95% CI: 1.64-2.86), respectively. The overall CAHMA significantly increased in the midwest, south, and west, while the northeast did not show any significant change over the past 12 years. The west and south showed troubling trends for overall CAHMA; in the west, hospitalizations increased dramatically over the last 12 years (P < 0.0001). Similarly, in the South, CAHMA increased 3-fold over the last 12 years (P < 0.0001). The adjusted and unadjusted PRs are shown in more detail in Table 2.
Discussion
According to the 2016 National Drug Threat Survey, methamphetamine is considered the most significant drug threat after opioids. In 2021, the U.S. Congress introduced the Methamphetamine Response Act of 2021.21 Pulmonary hypertension, heart failure, myocardial infarction, and cardiomyopathy have been reported to be associated with methamphetamine use. Additionally, methamphetamine is known to be cardiotoxic through multiple proposed mechanisms.22, 23, 24, 25, 26 Previous studies have reported rising cardiovascular complications related to methamphetamine use.6,27, 28, 29, 30, 31, 32, 33, 34 Another study showed the geographic and social disparities for heart failure among methamphetamine users using 2002 to 2014 NIS data5 but didn't show any trend analysis. Based on our knowledge, this is the first study that comprehensively analyzes the geographical and demographic trend for CAHMA across the United States, utilizing 13 years of most recent hospital admission data.
We found that the prevalence of cardiomyopathy among methamphetamine users had increased by 231%, and all-cause mortality increased by 195% between 2008 and 2020. Demographically, cardiomyopathy among methamphetamine users occurs predominantly in middle-aged patients and men and tends to be concentrated more in the western region of the United States. Clinically, hypertension is the most common comorbidity of cardiomyopathy reported among methamphetamine users (54%), followed by diabetes (24%).
The current literature shows that methamphetamine use is consistently rising globally35,36,. Additionally, several reports have shown an increasing trend of methamphetamine-associated CVD in the United States, but analysis related to geographical variation is limited. For instance, methamphetamine-associated heart failure hospitalizations have been reported much more frequently in the western states compared to the rest of the country. Consistently, our study revealed that methamphetamine use demonstrated a remarkable increase between 2008 and 2020 in the western region. Based on 2008 to 2020 hospital admission data, cardiomyopathy-related hospital admission among methamphetamine users increased by 530% in the western region and 200% in the southern region of the United States. In parallel, methamphetamine users' hospital admissions are more prevalent in the western and southern regions of the United States, indicating a potential correlation between methamphetamine use and cardiomyopathy. Furthermore, the geographic trend analysis signals the increased availability of methamphetamine in the west and south regions of the United States, and hospital admissions for CAHMA are likely to increase soon.
Previous studies highlighted the unique demographics of methamphetamine-associated CVD compared to the traditional risk factors. Our study showed that despite the vast number of hospital admissions due to methamphetamine use in whites (∼1,021%) compared to other races, the PR of CAHMA is higher in non-Hispanic Whites, non-Hispanic Native Americans, and Hispanics compared to non-Hispanic Blacks, consistent with previous studies. Between 2008 and 2020, there was a significant surge in CAHMA among non-Hispanic Asian or non-Hispanic Pacific Islanders, non-Hispanic Native Americans, or Hispanics, with a more than 500% increase, while non-Hispanic Whites and blacks showed around 250% increase. These findings' correlation with health care inequalities should be explored in greater detail. Medicare was prominent among methamphetamine and CAHMA patients, and Medicaid was prominent among cardiomyopathy patients. We have demonstrated that patients enrolled in Medicare insurance were more than 4 times more likely to have cardiomyopathy and be methamphetamine users compared to patients enrolled in other insurance. Reliance on the Medicare insurance system is predominant among low socioeconomic groups and certain ethnicities, which may have an adverse effect on the prognosis of cardiomyopathy among methamphetamine users and other methamphetamine-associated CVDs.
Several studies have demonstrated that methamphetamine-associated heart failure affects a younger population (35-60 years old, with a mean age of 48) hospitalized for methamphetamine-use-associated heart failure and consistent with previous findings that showed most methamphetamine users are 26 to 64 years old, with a mean age of ∼42 years. Compared to nonusers, more than half of cardiomyopathy among methamphetamine users affects the younger population (41-64 vs >65 years). Additionally, younger methamphetamine users (26-40 years old) showed a 3-fold increase in CAH compared to nonusers. The trend of methamphetamine use and its adverse impact on cardiovascular health, particularly among younger individuals, serves as a stark reminder of the need for preventive measures and effective treatment options.
The sex disparity associated with methamphetamine-associated CVD has demonstrated that methamphetamine use affects men more often than women.37, 38, 39 Based on hospital admission data, we found hospital admission for CAH among methamphetamine users increased by 345% for men and 123% for women. Despite the observed upward trend in hospital admissions for both sexes, the risk of hospital admissions was 0.99 times lower in women than in men. It is important to notice that some animal studies suggest that female hearts are more susceptible to methamphetamine-induced transcriptomic alteration; however, these changes seem to be transient after drug discontinuation.40 However, further research is needed to fully understand the gender-specific effects of methamphetamine use on cardiovascular health.
Study strengths and limitations
NIS data has both its limitations and its strengths. The strengths of NIS data are: 1) it captures a large sample size of hospitalization data that provide robust sample size to perform prevalence and trend analysis; 2) It provides multiple years of hospitalization data to perform national and regional level estimates; 3) NIS data provides an insight of hospital utilization and outcome at national level in the United States; 4) NIS data uses standardized diagnosis coding and classification system that provides the opportunity to produce a consistent and reproducible trend analysis for different disease-related hospital utilization at different time points. On the limitations: 1) the NIS data only captures community-level hospital information but no other types of hospitals such as long-term care or emergency department visits; 2) NIS data doesn't provide any individual-level information which limits detailed patient-level data analysis or adjust for confounding variables; 3) we cannot identify unique patients in the NIS database, and no postdischarge information is available; 4) methamphetamine use diagnosis mechanism is not available; 5) in the analysis, we focused on the prevalence and trends of clinical CAHMA, and doesn't discriminate based on any other biomarkers. These data don't identify the route of methamphetamine use (injection, smoking, or any other way). Other limitations of the NIS database have been explained previously in several studies.17 In this study, we focused on CAHMA. Previous studies showed an association between heart failure with tobacco, marijuana, alcohol, and cocaine abuse.10 Increasing emergency health record data may be helpful for better insight into the trend and identifying outcomes related to methamphetamine abuse.9,6
Conclusions
CAHMA have increased significantly in the United States. Men, Hispanic and non-Hispanic Asian population, age groups 41 to 64 years. and western regions showed a higher proportional increase highlighting gender-based, racial/ethnic, and regional disparities in methamphetamine use and associated cardiomyopathy-related hospitalizations over the study period. Further research is warranted to identify high-risk populations and develop strategies to prevent and mitigate CVD among methamphetamine users.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: The rise in cardiomyopathy-related hospital admissions among methamphetamine users in the United States requires understanding patterns, trends, and variations. Clinicians must be knowledgeable about these issues to effectively manage patients. Understanding the specific risks and complications associated with methamphetamine use is crucial for developing appropriate treatment plans.
TRANSLATIONAL OUTLOOK IMPLICATIONS: Further research is needed to develop preventive and mitigation strategies for cardiovascular disease among methamphetamine users, involving interdisciplinary collaboration. This could involve tailoring interventions to high-risk groups based on geographical, socioeconomic, and demographic variations, allowing health care professionals and public health experts to design targeted programs and policies.
Funding support and author disclosures
The National Institutes of Health grants supported this work: R01HL145753, R01HL145753-01S1, and R01HL145753-03S1; LSUHSC-S CCDS Finish Line Award, COVID-19 Research Award, and LARC Research Award to Dr Bhuiyan; Jane Cheever Powell Foundation for Cardiovascular Research Related to Gender and Isolation, LSUHS to Dr Bailey; and Institutional Development Award (IDeA) from the National Institutes of General Medical Sciences of the NIH under grant number P20GM121307 and R01HL149264 to Dr Kevill; NIH R01HL098435, R01HL133497 to Dr Orr. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Supplementary data
References
- 1.Xing D., Horan T., Bhuiyan M.S., et al. Social-geographic disparities in suicidal ideations among methamphetamine users in the USA. Psychiatr Res. 2023;329 doi: 10.1016/j.psychres.2023.115524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panenka W.J., Procyshyn R.M., Lecomte T., et al. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129(3):167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Bhuiyan M.S., Faisal A.S., Goeders N., et al. Disparities in trend and prevalence of methamphetamine-associated cardiomyopathy in the USA. J Am Coll Cardiol. 2023;81(18):1881–1883. doi: 10.1016/j.jacc.2023.03.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazumder S., Khan M.T.F., Bhuiyan M.A.N., Kiser H. Historical trends of admitted patients by selected substances and their significant patient's level factors. Addict Behav. 2020;109 doi: 10.1016/j.addbeh.2020.106478. [DOI] [PubMed] [Google Scholar]
- 5.Dickson S.D., Thomas I.C., Bhatia H.S., et al. Methamphetamine-associated heart failure hospitalizations across the United States: geographic and social disparities. J Am Heart Assoc. 2021;10(16) doi: 10.1161/JAHA.120.018370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diercks D.B., Fonarow G.C., Kirk J.D., et al. Illicit stimulant use in a United States heart failure population presenting to the emergency department (from the acute decompensated heart failure national Registry emergency module) Am J Cardiol. 2008;102(9):1216–1219. doi: 10.1016/j.amjcard.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 7.Batra V., Murnane K.S., Knox B., et al. Early onset cardiovascular disease related to methamphetamine use is most striking in individuals under 30: a retrospective chart review. Addict Behav Rep. 2022;15 doi: 10.1016/j.abrep.2022.100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran L., Nah G. Clinical correlates and outcomes of methamphetamine-associated cardiovascular diseases in hospitalized patients in California. J Am Heart Assoc. 2022;11(16) doi: 10.1161/jaha.121.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards J.R., Harms B.N., Kelly A., Turnipseed S.D. Methamphetamine use and heart failure: prevalence, risk factors, and predictors. Am J Emerg Med. 2018;36(8):1423–1428. doi: 10.1016/j.ajem.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura M., Bhatia H., Ma J., et al. The impact of substance abuse on heart failure hospitalizations. Am J Med. 2020;133(2):207–213.e1. doi: 10.1016/j.amjmed.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura M., Ma J., Fox S., et al. Characteristics and outcomes of methamphetamine abuse among veterans with heart failure. Am J Cardiol. 2019;124(6):907–911. doi: 10.1016/j.amjcard.2019.05.068. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Yehuda O., Siecke N. American College of Cardiology Foundation; 2018. Crystal Methamphetamine: A Drug and Cardiovascular Epidemic; pp. 219–221. [DOI] [PubMed] [Google Scholar]
- 13.Richardson S., Lin T., Li Y., et al. Guidance for use of weights: an analysis of different types of weights and their implications when using SAS PROCs. Gen Psychiatr. 2019;32(1) doi: 10.1136/gpsych-2018-100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang W., He H., Tu X.M. CRC Press; 2023. Applied Categorical and Count Data Analysis. [Google Scholar]
- 15.Barros A.J., Hirakata V.N. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3(1):1–13. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz-Quijano F.A. A simple method for estimating relative risk using logistic regression. BMC Med Res Methodol. 2012;12:1–6. doi: 10.1186/1471-2288-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khera R., Krumholz H.M. With great power comes great responsibility: big data research from the national inpatient sample. Circ Cardiovasc Qual Outcomes. 2017;10(7) doi: 10.1161/CIRCOUTCOMES.117.003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khera R., Pandey A., Kumar N., et al. Variation in hospital use and outcomes associated with pulmonary artery catheterization in heart failure in the United States. Circ Heart Fail. 2016;9(11) doi: 10.1161/CIRCHEARTFAILURE.116.003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khera R., Angraal S., Couch T., et al. Adherence to methodological standards in research using the national Inpatient sample. JAMA. 2017;318(20):2011–2018. doi: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overview of the national (nationwide) inpatient sample (NIS). Accessed February 8, 2024. https://hcup-us.ahrq.gov/nisoverview.jsp
- 21.Congress . 2022. S.854 - Methamphetamine Response Act of 2021. 117th Congress (2021-2022) Accessed February 8, 2024. https://www.congress.gov/bill/117th-congress/senate-bill/854#:∼:text=Shown%20Here%3A-,Public%20Law%20No%3A%20117%2D99,(03%2F14%2F2022)&text=This%20bill%20designates%20methamphetamine%20as,implement%20a%20methamphetamine%20response%20plan. [Google Scholar]
- 22.Kevil C.G., Goeders N.E., Woolard M.D., et al. Methamphetamine use and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2019;39(9):1739–1746. doi: 10.1161/ATVBAHA.119.312461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdullah C.S., Remex N.S., Aishwarya R., et al. Mitochondrial dysfunction and autophagy activation are associated with cardiomyopathy developed by extended methamphetamine self-administration in rats. Redox Biol. 2022;58 doi: 10.1016/j.redox.2022.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleckenstein A.E., Volz T.J., Riddle E.L., Gibb J.W., Hanson G.R. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 25.Lord K.C., Shenouda S.K., McIlwain E., Charalampidis D., Lucchesi P.A., Varner K.J. Oxidative stress contributes to methamphetamine-induced left ventricular dysfunction. Cardiovasc Res. 2010;87(1):111–118. doi: 10.1093/cvr/cvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdullah C.S., Aishwarya R., Alam S. Methamphetamine induces cardiomyopathy by Sigmar1 inhibition-dependent impairment of mitochondrial dynamics and function. Commun Biol. 2020;3(1):682. doi: 10.1038/s42003-020-01408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manja V., Nrusimha A., Gao Y., et al. Methamphetamine-associated heart failure: a systematic review of observational studies. Heart. 2023;109(3):168–177. doi: 10.1136/heartjnl-2022-321610. [DOI] [PubMed] [Google Scholar]
- 28.Zhao S.X., Deluna A., Kelsey K., et al. Socioeconomic burden of rising methamphetamine-associated heart failure hospitalizations in California from 2008 to 2018. Circ Cardiovasc Qual Outcomes. 2021;14(7) doi: 10.1161/CIRCOUTCOMES.120.007638. [DOI] [PubMed] [Google Scholar]
- 29.Schurer S., Klingel K., Sandri M., et al. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine-associated cardiomyopathy. JACC Heart Fail. 2017;5(6):435–445. doi: 10.1016/j.jchf.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Sliman S., Waalen J., Shaw D. Methamphetamine-associated congestive heart failure: increasing prevalence and relationship of clinical outcomes to continued use or abstinence. Cardiovasc Toxicol. 2016;16(4):381–389. doi: 10.1007/s12012-015-9350-y. [DOI] [PubMed] [Google Scholar]
- 31.Won S., Hong R.A., Shohet R.V., Seto T.B., Parikh N.I. Methamphetamine-associated cardiomyopathy. Clin Cardiol. 2013;36(12):737–742. doi: 10.1002/clc.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamanian R.T., Hedlin H., Greuenwald P., et al. Features and outcomes of methamphetamine-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197(6):788–800. doi: 10.1164/rccm.201705-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeo K.K., Wijetunga M., Ito H., et al. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med. 2007;120(2):165–171. doi: 10.1016/j.amjmed.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Richards J.R., Harms B.N., Kelly A., Turnipseed S.D. Methamphetamine use and heart failure: prevalence, risk factors, and predictors. Am J Emerg Med. 2018;36(8):1423–1428. doi: 10.1016/j.ajem.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Jones C.M., Compton W.M., Mustaquim D. Patterns and characteristics of methamphetamine use among adults - United States, 2015-2018. MMWR Morb Mortal Wkly Rep. 2020;69(12):317–323. doi: 10.15585/mmwr.mm6912a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darke S., Duflou J., Kaye S. Prevalence and nature of cardiovascular disease in methamphetamine-related death: a national study. Drug Alcohol Depend. 2017;179:174–179. doi: 10.1016/j.drugalcdep.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Reddy P.K., Chau E., Patel S.V., Yang K., Ng T.M., Elkayam U. Characteristics of methamphetamine-associated cardiomyopathy and the impact of methamphetamine use on cardiac dysfunction. Am J Cardiol. 2021;154:86–91. doi: 10.1016/j.amjcard.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Thomas I.C., Nishimura M., Ma J., et al. Clinical characteristics and outcomes of patients with heart failure and methamphetamine abuse. J Card Fail. 2020;26(3):202–209. doi: 10.1016/j.cardfail.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Marcinko M.C., Darrow A.L., Tuia A.J., Shohet R.V. Sex influences susceptibility to methamphetamine cardiomyopathy in mice. Physiol Rep. 2019;7(6) doi: 10.14814/phy2.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavva H., Brazeau D.A., Denvir J., et al. Methamphetamine-induced changes in myocardial gene transcription are sex-dependent. BMC Genom. 2021;22(1):1–16. doi: 10.1186/s12864-021-07561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.