Abstract
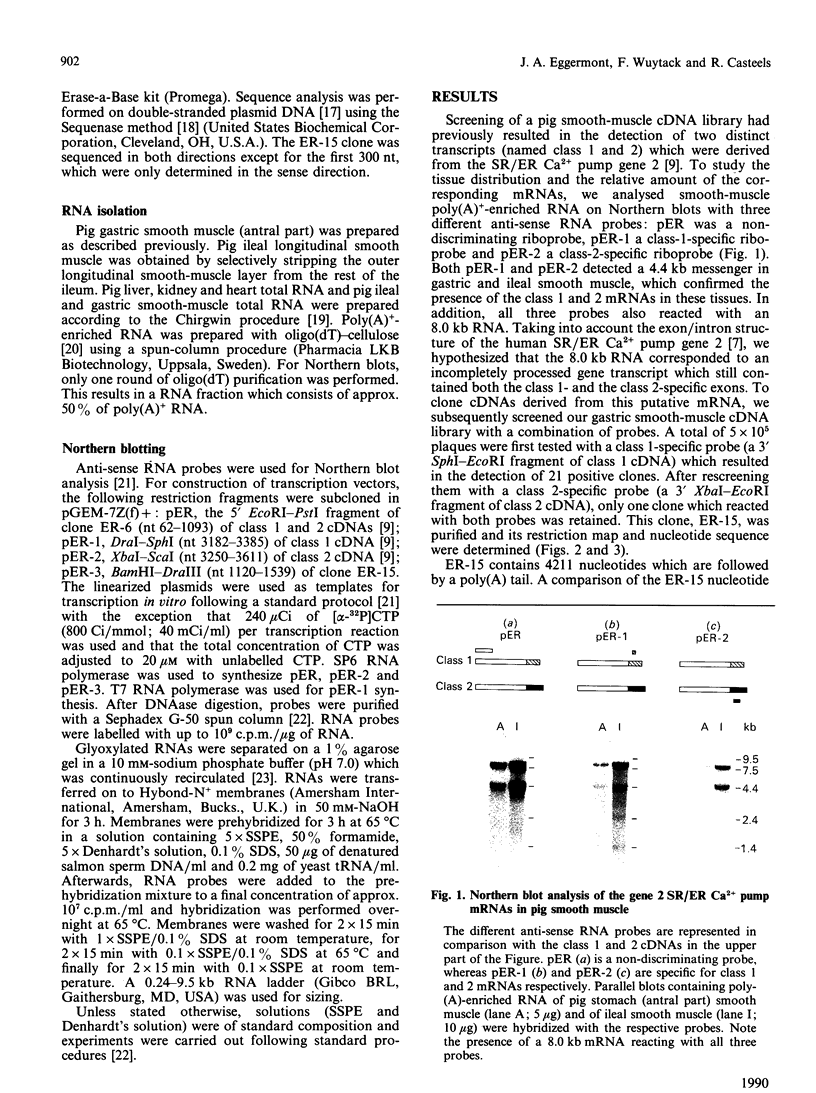
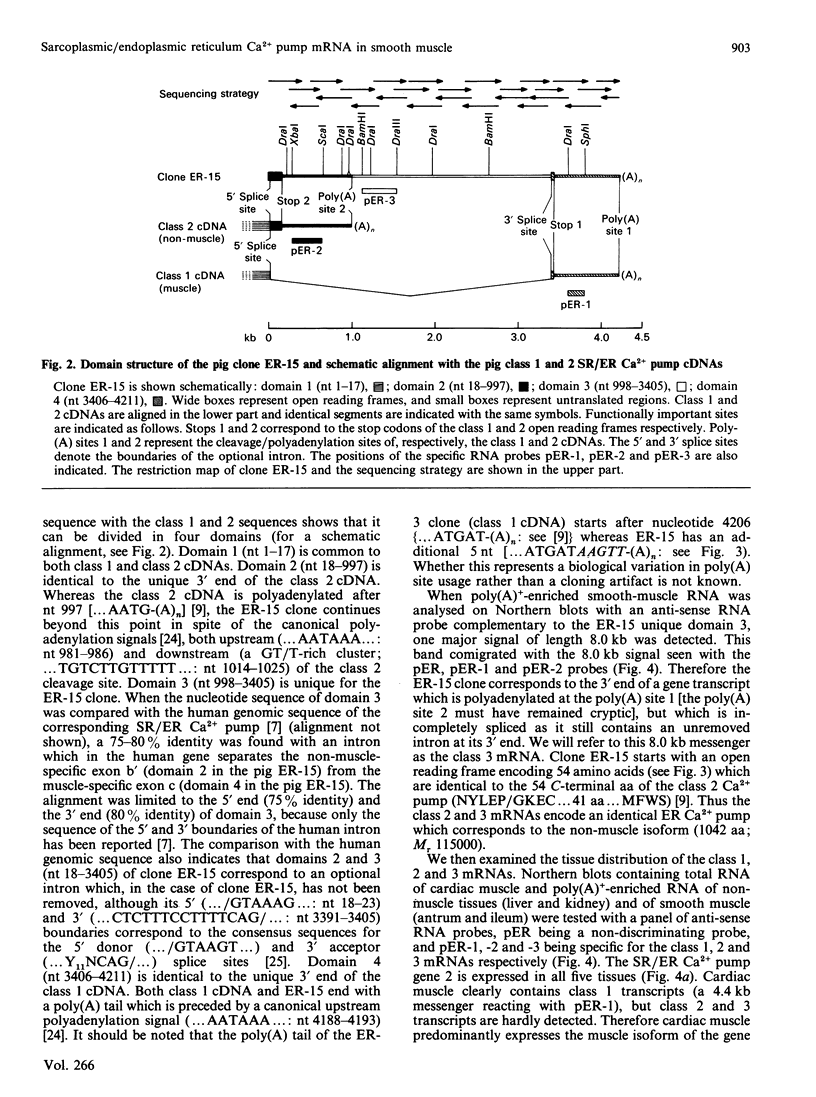
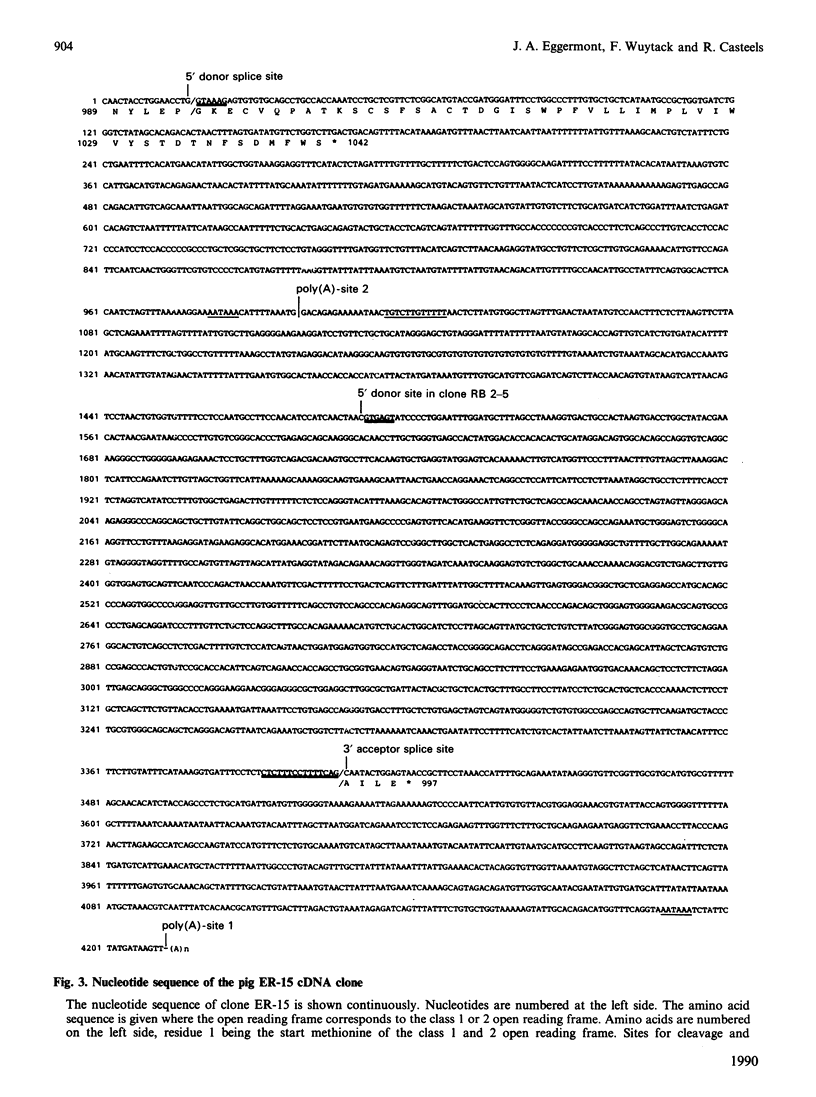
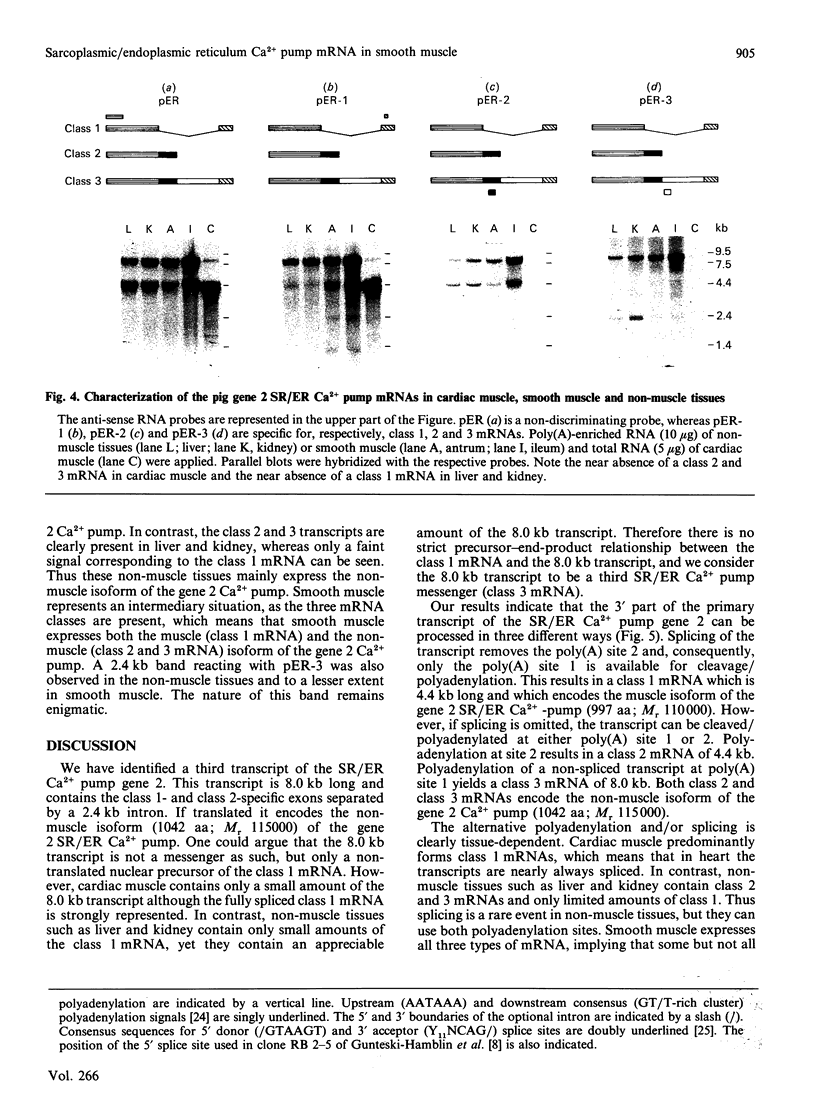
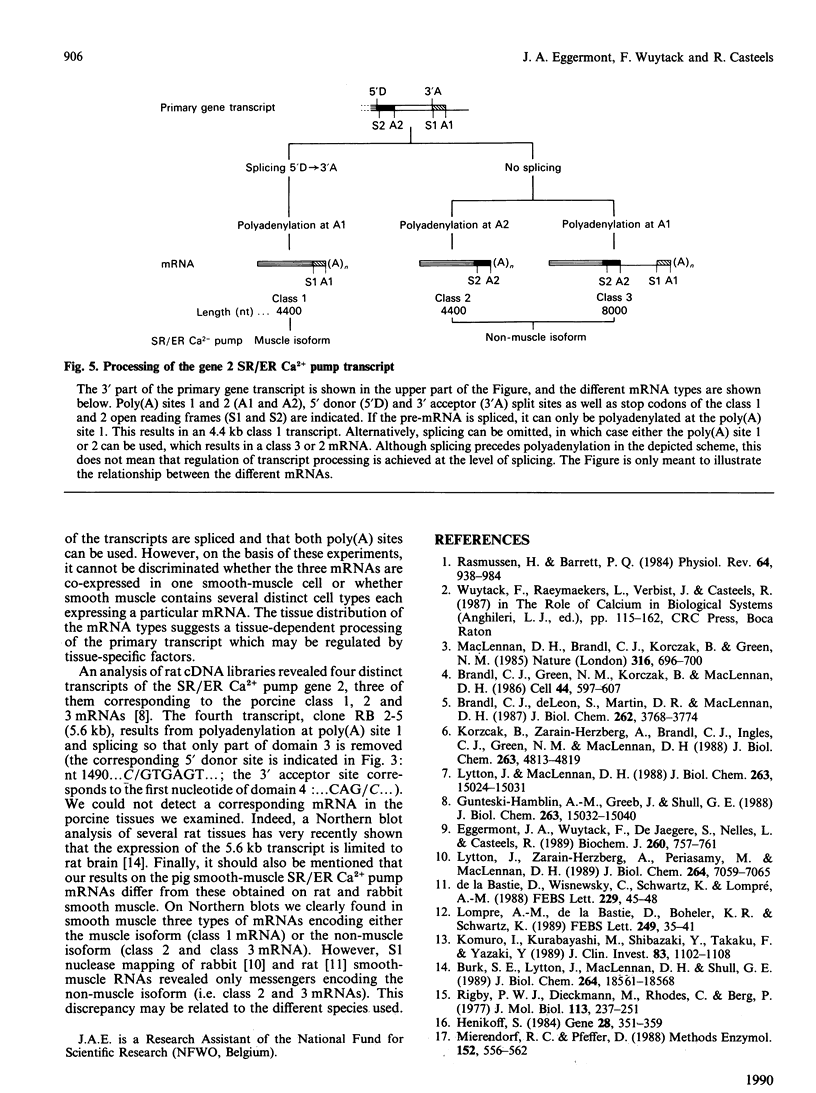
The gene 2 sarcoplasmic/endoplasmic-reticulum (SR/ER) Ca2+ pump is expressed in slow skeletal and cardiac muscle, smooth muscle and non-muscle tissues. We have analysed the gene 2 Ca2+ pump mRNAs using a panel of anti-sense RNA probes which recognize either the muscle (class 1) or the non-muscle (class 2) transcript, or both. In pig smooth muscle, we confirmed the presence of the class 1 and class 2 mRNAs of 4.4 kb length and we also detected a third mRNA of 8.0 kb which reacted with both the class 1 and class 2 riboprobes. A 4.2 kb cDNA corresponding to the 3' part of the 8.0 kb mRNA was cloned from a pig gastric smooth muscle cDNA library. Nucleotide sequence analysis of this clone revealed that the 8.0 kb mRNA (class 3 transcript) contained both the non-muscle-specific and the muscle-specific exons separated by a 2.4 kb intron which has not been removed. The class 3-mRNA-encoded SR/ER Ca2+ pump is identical to the class 2-encoded non-muscle isoform. Northern blot analysis demonstrated that, in cardiac muscle, the class 1 mRNA (encoding the muscle isoform) is the predominant messenger, whereas in non-muscle tissues the class 2 and 3 mRNAs (encoding the non-muscle isoform) predominate. In smooth muscle all three mRNA types are present. The tissue distribution of the mRNA types suggests a tissue-dependent processing of the primary transcript of the sarcoplasmic/endoplasmic reticulum Ca2+ pump gene 2.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., deLeon S., Martin D. R., MacLennan D. H. Adult forms of the Ca2+ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J Biol Chem. 1987 Mar 15;262(8):3768–3774. [PubMed] [Google Scholar]
- Burk S. E., Lytton J., MacLennan D. H., Shull G. E. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989 Nov 5;264(31):18561–18568. [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Eggermont J. A., Wuytack F., De Jaegere S., Nelles L., Casteels R. Evidence for two isoforms of the endoplasmic-reticulum Ca2+ pump in pig smooth muscle. Biochem J. 1989 Jun 15;260(3):757–761. doi: 10.1042/bj2600757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunteski-Hamblin A. M., Greeb J., Shull G. E. A novel Ca2+ pump expressed in brain, kidney, and stomach is encoded by an alternative transcript of the slow-twitch muscle sarcoplasmic reticulum Ca-ATPase gene. Identification of cDNAs encoding Ca2+ and other cation-transporting ATPases using an oligonucleotide probe derived from the ATP-binding site. J Biol Chem. 1988 Oct 15;263(29):15032–15040. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kurabayashi M., Shibazaki Y., Takaku F., Yazaki Y. Molecular cloning and characterization of a Ca2+ + Mg2+-dependent adenosine triphosphatase from rat cardiac sarcoplasmic reticulum. Regulation of its expression by pressure overload and developmental stage. J Clin Invest. 1989 Apr;83(4):1102–1108. doi: 10.1172/JCI113989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczak B., Zarain-Herzberg A., Brandl C. J., Ingles C. J., Green N. M., MacLennan D. H. Structure of the rabbit fast-twitch skeletal muscle Ca2+-ATPase gene. J Biol Chem. 1988 Apr 5;263(10):4813–4819. [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Lompre A. M., de la Bastie D., Boheler K. R., Schwartz K. Characterization and expression of the rat heart sarcoplasmic reticulum Ca2+-ATPase mRNA. FEBS Lett. 1989 May 22;249(1):35–41. doi: 10.1016/0014-5793(89)80010-9. [DOI] [PubMed] [Google Scholar]
- Lytton J., MacLennan D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988 Oct 15;263(29):15024–15031. [PubMed] [Google Scholar]
- Lytton J., Zarain-Herzberg A., Periasamy M., MacLennan D. H. Molecular cloning of the mammalian smooth muscle sarco(endo)plasmic reticulum Ca2+-ATPase. J Biol Chem. 1989 Apr 25;264(12):7059–7065. [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Mierendorf R. C., Pfeffer D. Direct sequencing of denatured plasmid DNA. Methods Enzymol. 1987;152:556–562. doi: 10.1016/0076-6879(87)52061-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Bastie D., Wisnewsky C., Schwartz K., Lompré A. M. (Ca2+ + Mg2+)-dependent ATPase mRNA from smooth muscle sarcoplasmic reticulum differs from that in cardiac and fast skeletal muscles. FEBS Lett. 1988 Feb 29;229(1):45–48. doi: 10.1016/0014-5793(88)80794-4. [DOI] [PubMed] [Google Scholar]