Abstract
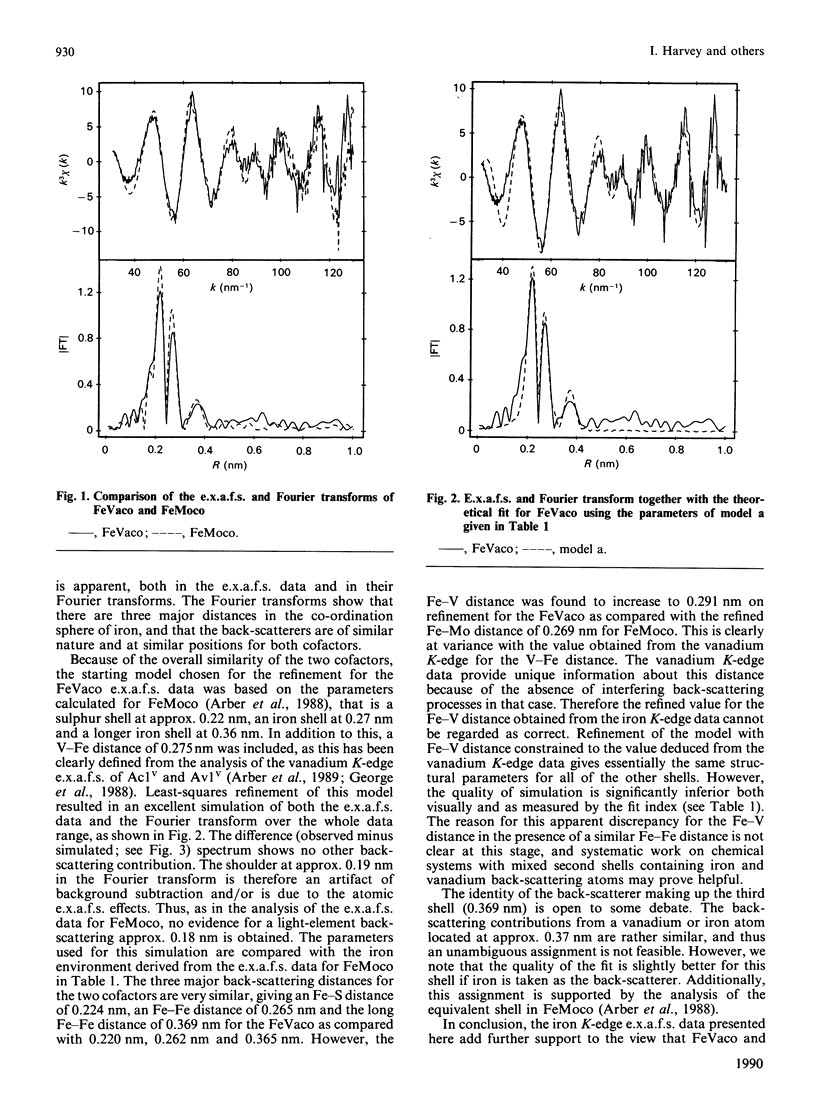
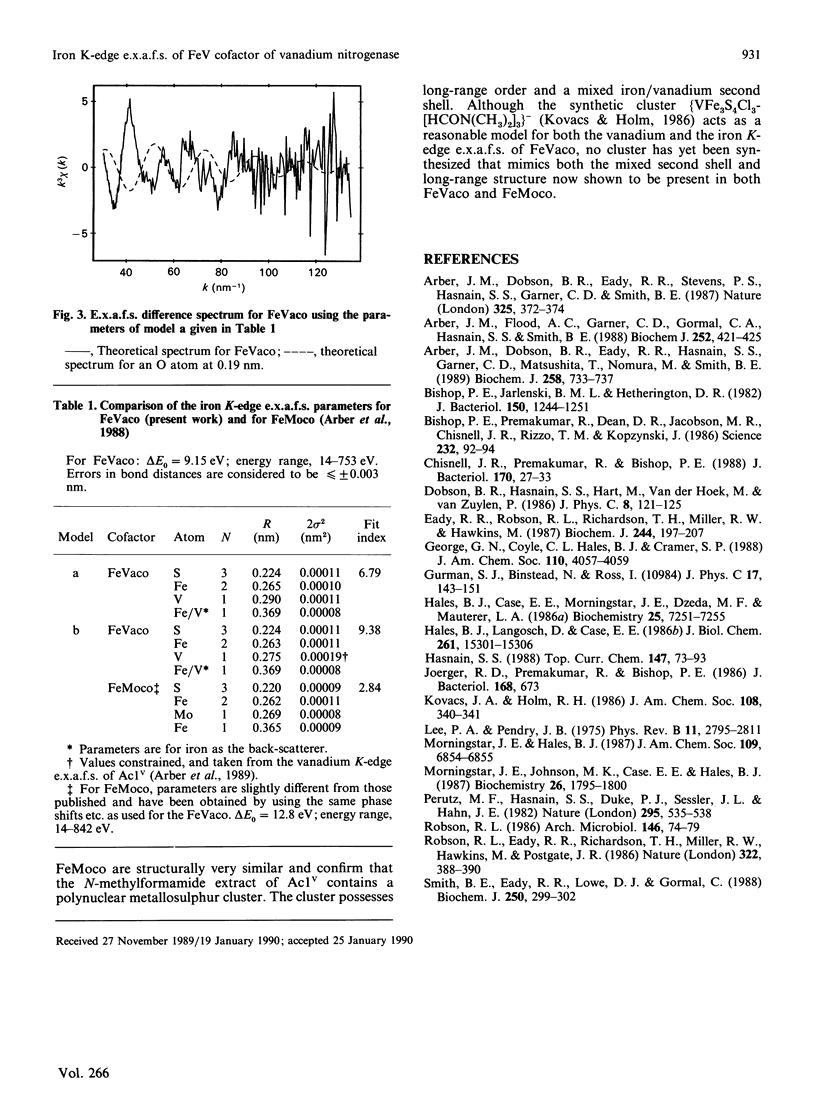
Iron K-edge e.x.a.f.s. data for the iron-vanadium cofactor (FeVaco) from Azotobacter chroococcum vanadium nitrogenase reported here provide further evidence for the structural similarity between this and the iron-molybdenum nitrogenase cofactor (FeMoco) from Klebsiella pneumoniae molybdenum nitrogenase [Arber, Flood, Garner, Gormal, Hasnain & Smith (1988) Biochem. J. 252, 421-425]. The e.x.a.f.s. data are consistent with the vanadium being present in a V-Fe-S cluster, thus confirming that the N-methylformamide extract of the VFe protein component of A. chroococcum vanadium nitrogenase does indeed contain a polynuclear metal-sulphur cluster. Additionally, a long Fe-Fe distance is observed as 0.369 nm, demonstrating the presence of a long-range order in the cluster.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber J. M., Dobson B. R., Eady R. R., Hasnain S. S., Garner C. D., Matsushita T., Nomura M., Smith B. E. Vanadium K-edge X-ray-absorption spectroscopy of the functioning and thionine-oxidized forms of the VFe-protein of the vanadium nitrogenase from Azotobacter chroococcum. Biochem J. 1989 Mar 15;258(3):733–737. doi: 10.1042/bj2580733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber J. M., Flood A. C., Garner C. D., Gormal C. A., Hasnain S. S., Smith B. E. Iron K-edge X-ray absorption spectroscopy of the iron-molybdenum cofactor of nitrogenase from Klebsiella pneumoniae. Biochem J. 1988 Jun 1;252(2):421–425. doi: 10.1042/bj2520421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Expression of an alternative nitrogen fixation system in Azotobacter vinelandii. J Bacteriol. 1982 Jun;150(3):1244–1251. doi: 10.1128/jb.150.3.1244-1251.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Premakumar R., Dean D. R., Jacobson M. R., Chisnell J. R., Rizzo T. M., Kopczynski J. Nitrogen Fixation by Azotobacter vinelandii Strains Having Deletions in Structural Genes for Nitrogenase. Science. 1986 Apr 4;232(4746):92–94. doi: 10.1126/science.232.4746.92. [DOI] [PubMed] [Google Scholar]
- Chisnell J. R., Premakumar R., Bishop P. E. Purification of a second alternative nitrogenase from a nifHDK deletion strain of Azotobacter vinelandii. J Bacteriol. 1988 Jan;170(1):27–33. doi: 10.1128/jb.170.1.27-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Robson R. L., Richardson T. H., Miller R. W., Hawkins M. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the VFe protein. Biochem J. 1987 May 15;244(1):197–207. doi: 10.1042/bj2440197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales B. J., Case E. E., Morningstar J. E., Dzeda M. F., Mauterer L. A. Isolation of a new vanadium-containing nitrogenase from Azotobacter vinelandii. Biochemistry. 1986 Nov 18;25(23):7251–7255. doi: 10.1021/bi00371a001. [DOI] [PubMed] [Google Scholar]
- Hales B. J., Langosch D. J., Case E. E. Isolation and characterization of a second nitrogenase Fe-protein from Azotobacter vinelandii. J Biol Chem. 1986 Nov 15;261(32):15301–15306. [PubMed] [Google Scholar]
- Joerger R. D., Premakumar R., Bishop P. E. Tn5-induced mutants of Azotobacter vinelandii affected in nitrogen fixation under Mo-deficient and Mo-sufficient conditions. J Bacteriol. 1986 Nov;168(2):673–682. doi: 10.1128/jb.168.2.673-682.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morningstar J. E., Johnson M. K., Case E. E., Hales B. J. Characterization of the metal clusters in the nitrogenase molybdenum-iron and vanadium-iron proteins of Azotobacter vinelandii using magnetic circular dichroism spectroscopy. Biochemistry. 1987 Apr 7;26(7):1795–1800. doi: 10.1021/bi00381a001. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Hasnain S. S., Duke P. J., Sessler J. L., Hahn J. E. Stereochemistry of iron in deoxyhaemoglobin. Nature. 1982 Feb 11;295(5849):535–538. doi: 10.1038/295535a0. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Eady R. R., Lowe D. J., Gormal C. The vanadium-iron protein of vanadium nitrogenase from Azotobacter chroococcum contains an iron-vanadium cofactor. Biochem J. 1988 Feb 15;250(1):299–302. doi: 10.1042/bj2500299. [DOI] [PMC free article] [PubMed] [Google Scholar]


