ABSTRACT
Multiple sclerosis (MS) is a chronic and progressive autoimmune disease of the central nervous system (CNS), with both genetic and environmental factors contributing to the pathobiology of the disease. While human leukocyte antigen (HLA) genes have emerged as the strongest genetic factor, consensus on environmental risk factors are lacking. Recently, trillions of microbes residing in our gut (microbiome) have emerged as a potential environmental factor linked with the pathobiology of MS as PwMS show gut microbial dysbiosis (altered gut microbiome). Thus, there has been a strong emphasis on understanding the factors (host and environmental) regulating the composition of the gut microbiota and the mechanism(s) through which gut microbes contribute to MS disease, especially through immune system modulation. A better understanding of these interactions will help harness the enormous potential of the gut microbiota as a therapeutic approach to treating MS.
KEYWORDS: Multiple sclerosis, gut microbiome, diet, fiber, inflammation, therapeutic, phytoestrogen, short-chain fatty acids, tryptophan, LPS
1. Pathobiology of multiple sclerosis
Multiple sclerosis (MS), a chronic autoimmune disorder of the central nervous system (CNS) affecting an estimated 2.8 million individuals globally, is a significant contributor to neurological disability in young adults.1,2 MS is categorized into various subtypes based on radiological and pathological findings, each with distinct clinical courses and prognoses.3 The most common form, relapsing-remitting MS (RRMS), is characterized by episodes of neurological symptoms followed by periods of remission. The progressive forms of MS include primary progressive MS (PPMS) and secondary progressive MS (SPMS), where disability steadily accumulates over time.3 Additionally, radiologically isolated syndrome (RIS) and clinically isolated syndrome (CIS) are recognized as pre-MS stages, with RIS characterized by radiological evidence of demyelination without clinical symptoms and CIS presenting with a first clinical episode suggestive of demyelination but not yet fulfilled the criteria for a definitive MS diagnosis.3
MS pathogenesis results from multifactorial etiology involving genetic and environmental factors.4,5 The Human Leukocyte Antigen (HLA) gene complex, which plays a critical role in the immune system, has emerged as a critical genetic risk factor in MS, with certain HLA-class II genes having been linked with an increased risk of developing MS.6,7 In addition to the HLA gene, other genetic variants have also been implicated in MS susceptibility, including genes involved in immune regulation, myelin formation, and neuronal signaling.8 However, studies of monozygotic twins have shown that genetic factors contribute only ~30% of the disease risk,9 with the rest linked to environmental factors. Environmental factors, such as gut microbiota, vitamin D deficiency, smoking, diet, and infections such as Epstein–Barr virus, have been linked with MS.5 In the last decade, gut microbiota dysbiosis, an imbalance in the composition and function of gut bacteria, has emerged as a potential environmental factor contributing to the pathobiology of MS.10
The underlying pathophysiology of MS involves a dysregulated immune response, primarily driven by the infiltration of T cells, B cells, and macrophages into the central nervous system (CNS). Activated T cells, particularly T-helper 1 (Th1) and T-helper 17 (Th17) subsets, secrete pro-inflammatory cytokines such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), and IL-17, leading to inflammation, demyelination, and axonal damage.11,12 While the initial triggers of MS remain elusive, a prevailing hypothesis suggests that in genetically predisposed individuals, autoreactive CD4+ T cells targeting central nervous system (CNS) myelin antigens become activated in the periphery.11,12 These activated CD4+ T cells, secreting pro-inflammatory cytokines, breach the blood–brain barrier, a process facilitated by the interaction of alpha 4 beta 1 integrins on their surface with Vascular Cell Adhesion Molecule VCAM-1 expressed on endothelial cells.13 This critical interaction allows for the transmigration of these autoreactive T cells into the CNS, where they initiate an immune assault on the myelin sheath-insulating neuronal axons. This dysregulated immune response triggers a cascade of inflammation, recruiting additional immune cells such as B cells, macrophages, and neutrophils from the periphery and activating glial cells into pro-inflammatory phenotypes, culminating in demyelination and axonal damage.14 This pathological process manifests in a wide array of debilitating clinical symptoms, including optic neuritis, muscle weakness, sensory disturbances, fatigue, and cognitive impairment.
Under normal physiological conditions, robust immune regulatory mechanisms prevent inflammatory cascades that can lead to inflammation and demyelination in MS. Typically, autoreactive CD4+ T cells are held in check by immunoregulatory cells, such as regulatory CD4+ T cells expressing FoxP3 and/or IL-10 producing type 1 regulatory cells (Tr1).15 Additionally, other immune cells, such as CD8+ T cells, B cells, and NKT cells, can regulate autoreactive immune responses.15,16 However, in genetically predisposed individuals, environmental triggers can overcome these tolerance mechanisms, enabling autoreactive T cells to initiate pathogenic processes. The importance of CD4 and CD8 T cells in the pathobiology of MS is validated by a defective immunoregulatory response characterized by reduced number and/or function of regulatory immune cells during disease relapse.15,16
The gut microbiome communicates with the brain through various pathways, including neural and immune signaling. This bidirectional communication, known as the gut-brain axis, highlights the potential for gut bacteria and their metabolites to influence pathobiology of MS. Given the association between dysregulated immune response and MS, gut dysbiosis, which can influence immune regulation, may contribute to the development and exacerbation of MS. Disruptions in the gut microbial community can lead to increased intestinal permeability, allowing bacterial products and toxins to leak into the bloodstream, triggering the production of pro-inflammatory cytokines, and potentially facilitating immune cell infiltration into the CNS.17 Additionally, certain gut bacteria may directly modulate immune responses, promoting the development of autoreactive pro-inflammatory T cell subsets.18 Finally, gut bacteria produce a variety of metabolites such as short-chain fatty acids (SCFAs), tryptophan metabolites, phytoestrogen metabolites, and bile acids that can directly or indirectly impact the nervous system.19–22
This review will focus on how gut microbiota and their metabolites might contribute to the pathobiology of MS through the modulation of inflammatory responses in the periphery and CNS. Deciphering the intricate interplay between the pro-inflammatory immune response and the gut microbiome is paramount to a comprehensive understanding of MS pathobiology. This knowledge holds immense potential for harnessing gut microbiota manipulation as a therapeutic strategy to mitigate the debilitating effects of MS. Modulating the gut microbiome and dietary interventions may offer novel avenues for immunomodulation and disease management, ultimately improving the quality of life for individuals living with MS.
2. Gut microbiota and human health
The human gastrointestinal tract is colonized by trillions of diverse microorganisms, such as viruses, bacteria, and fungi, collectively called the gut microbiome.23 These highly diverse communities have the potential to influence human health systemically. The gut microbiome forms a symbiotic relationship with the host, where the host provides food and space for microbes to survive and grow while the microbes aid in maintaining the host’s health by helping with various physiological processes.24,25 A diverse gut microbiome develops in humans from birth until approximately 3 years of age; however, the specific microbes present can be altered by host-derived and environmental factors.26 Examples of host factors include molecules produced by intestinal epithelial cells, including mucus, antimicrobial peptides, immunoglobulin A (IgA), and MicroRNAs (miRNAs). Environmental factors like diet, lifestyle, medications, age, and delivery pattern can alter the gut microbiome’s composition.27 Changes in gut microbiota composition can perturb a balanced ecology characterized by reduced microbial richness, loss of beneficial microbes, and increases in pathobionts – commonly known as dysbiosis.28 This review will discuss the role of gut microbiota and diet as environmental factors in the pathobiology of MS.
The gut microbiome can affect various functions throughout the human body. Recent studies have noted that the gut microbiome can maintain bidirectional communication with the CNS.29,30 Through the vagus nerve, neuroendocrine system (ENS), and immune system, the CNS can directly or indirectly influence gut functions, such as nutrient uptake, gut permeability, and mucus production.31 On the other hand, the gut microbiome can communicate with the CNS through metabolite production that can act on the CNS directly or indirectly by activating immune cells that interact with it.21,22,32 Moreover, PwMS are reported to have symptoms such as constipation, diarrhea, and gastrointestinal (GI) discomfort.33–35 Although dysbiosis can be detected in these patients, there is no specific gut microbiome compositional signature for individual diseases, thus emphasizing the importance of better understanding how the gut microbiome may be linked to neurodegenerative diseases like MS.
3. Links between the gut microbiome and MS
Several studies, including from our groups, have shown that people with MS (PwMS) have gut dysbiosis characterized by a distinct gut microbiome compared to sex- and age-matched healthy controls.36–48 Specifically, PwMS show enrichment of gut bacteria such as Ruminococcus, Blautia, Dorea, Bifidobacterium, Bilophila, Sutterella, and Akkermansia (Table 1). Conversely, the genera Clostridium, Faecalibacterium, Eubacterium, Ruminococcus, Butyricimonas, Bacteroides, and Prevotella showed reduced abundance in PwMS (Table 1). These studies are further supported by the mouse model of MS, known as experimental autoimmune encephalomyelitis (EAE), of which the transplantation of MS patient gut bacteria to germ-free mice resulted in exacerbated EAE disease compared to healthy controls.36,38 Additionally, PwMS have also been shown to have distinct mycobiome (fungus).46,49
Table 1.
Summary of MS microbiome studies.
| MS type and sample size | Lower abundance in PwMS | Increased abundance in PwMS | Geographical location (Reference) |
|---|---|---|---|
| RRMS (n = 20) HC (n = 40) |
Bacteroides (B. stercoris, B. coprocola, B. coprophilus) Fecalibacterium sp. Prevotella (P. copri) Anaerostipes sp. Clostridium sp. Sutterella (S. wadsworthensis) |
Bifidobacterium sp. Streptococcus sp. Streptococcus thermophilus Eggerthella lenta |
Japan 43 |
| RRMS (n = 31) HC (n = 36) |
Prevotella sp. Parabacteroides sp. Adlercreutzia sp. Collinsella sp. sp. Lactobacillus |
Pedobacter sp. Pseudomonas sp. Mycoplasma sp. Haemophilus sp. Blautia sp. Dorea sp. |
USA 39 |
| RRMS (n = 60) HC (n = 43) |
Butyricimona sp. Prevotella sp. Parabacteroides sp. |
Methanobrevibacter sp. Akkermansia sp. |
USA 42 |
| RRMS (n = 30) HC (n = 14) |
Eubacterium eligens Prevotella copri uncultured Bacteroides sp. uncultured alpha Proteobacterium uncultured Pseudomonas sp |
Faecalibacterium sp. Ruminococcus sp. uncultured Oscillospiraceae sp. uncultured Blautia sp. Anaerostipes sp. Clostridium bolteae uncultured Dialister sp. Alistipes onderdonkii Bifidobacterium longum Coriobacterium sp |
UK 48 |
| RRMS (n = 71) HC (n = 71) |
Parabacteroides distasonis |
Akkermansia muciniphila Acinetobacter calcoaceticus |
USA 38 |
| RRMS (n = 24) HC (n = 25) |
Bifidobacterium longum Clostridium leptum Faecalibacterium prausnitzii Bacteroides thetaiotaomicron unclassified Escherichia Anaerostipes sp. Prevotella sp. |
unclassified Parabacteroides | USA 37 |
| RRMS (n = 19) HC (n = 17) |
Prevotella sp. | Streptococcus sp. | Italy 40 |
| Monozygotic twin pairs: MS (n = 34) Unaffected twin (n = 34) |
Adlercreutzia sp. | Akkermansia sp. | Germany 36 |
| POMS (n = 20) HC (n = 20) |
Pseudomonas Corrgata Haemophilus Influenzae Kochuria Sphingopyxis sp.QXT-31 |
Methanobrevibacter smithi Moribacter Mycoplasma bovoculi Diaphorobacter polyhydrobutyrativorans Methanobrevibacter millerae Arcanobacterium sp 2701 |
Canada 47 |
| RRMS (n = 20) HC (n = 30) |
Barnesiella sp. Odoribacter sp. Oscillospiracecae UCG 003 |
Blautia Sp Eggerthela sp. Hungatella sp. |
USA 46 |
| PwMS (n = 576) Paired HC (n = 1152) |
Faecalibacterium prausnitzii Blautia species |
Akkermansia muciniphila Ruthenibacterium lactatiformans Hungatella hathewayi Eisenbergiella tayi |
USA, Spain, UK, and Argentina 41 |
Abbreviations: RRMS, Relapsing Remitting Multiple Sclerosis (MS); HC, Healthy Control; POMS, Pediatric onset MS; PwMS, People with MS.
Although cross-sectional studies have helped establish a link between gut microbiota and MS, the question remains whether gut microbiota causes disease or disease causes dysbiosis. A recent study suggests a direct correlation between species richness and the number of disease relapses.44 The clinically non-active (non-relapsing) patients showed enrichment of Faecalibacterium prausnitzii, Gordonibacter urolithinfaciens, Anaerostipes hadrus, Gemmiger formicilis, and Roseburia inulinivorans compared to clinically active patients (who had at least one relapse in follow-up period). In contrast, the clinically active group showed enrichment of Methanobrevibacter smithii and Victivallis vadensis. Interestingly, bacterial species enriched in clinically active treatment-naïve cases were positively associated with circulating levels of proinflammatory cytokines IL-17A, IFN-γ, and TNF-α. Thus, this study strongly suggests that gut microbiota can directly contribute to the severity of MS disease. Data from MS microbiome studies are compiled in recent reviews10,50,51 and Table 1. In a study of pediatric PwMS, microbial alpha and beta diversities were not associated with relapses.52 However, they found that Butyricicoccus desmolans, Odoribacter splanchnic, Lacnhospiraceae NK4A136, and Ruminococcaceae species were associated with lower hazard to MS relapse while Blautia terrorism, Lachnoclostridium, Lachnospiraceae_UCG-004, and Coriobacteriales were associated with higher hazard to MS relapse.52 Although no significant associations of metabolic pathways to relapse were observed, super-pathways of L-tyrosine and L-phenylalanine biosynthesis were associated with a lower hazard of MRI outcomes. These studies imply that the gut microbiota is altered in relapses; however, further studies are required to determine whether microbiome modulation during relapses can alter the severity of RRMS outcomes in patients.
The altered gut microbiome observed in MS has fueled intense research interest in elucidating the factors shaping this microbial community and the mechanisms by which these microbes might influence MS pathogenesis (Figure 1).
Figure 1.
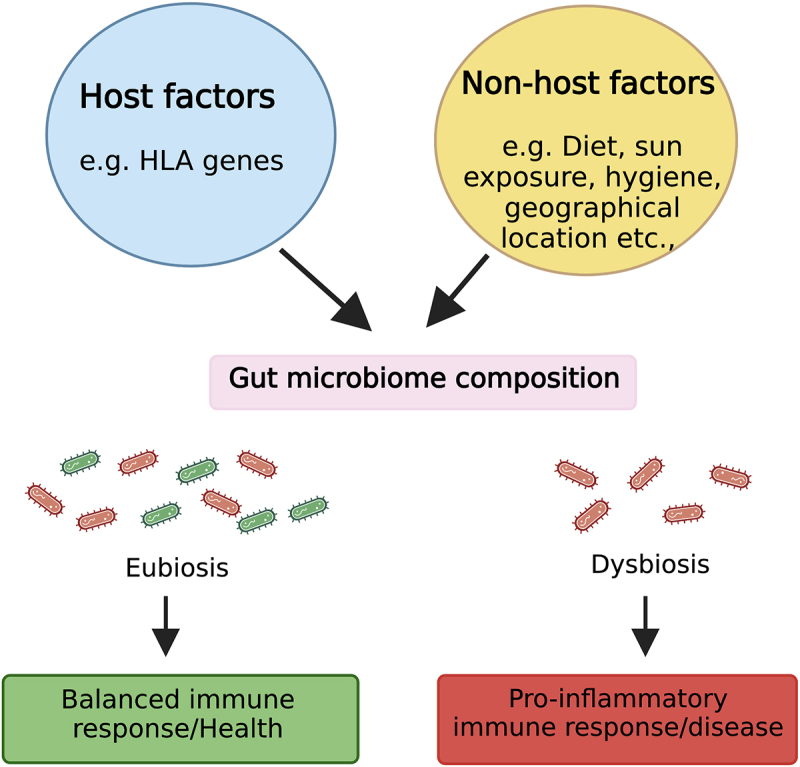
Factors affecting gut microbiota and a potential mechanism through which gut microbiota affects the host during health and disease. Host-specific factors such as host genetics and non-host factors like diet can influence gut microbiota composition. During homeostasis, an eubiotic gut microbiota maintains a diverse beneficial microbiota (symbiont) that induces a balanced immune response. However, during dysbiosis, there is depletion and/or enrichment of pro-inflammatory microbiota (pathobionts), which shift the balance between pro and anti-inflammatory responses toward an inflammatory phenotype linked with multiple diseases, including MS. Figure created with BioRender.com.
4. Factors affecting composition and function of gut microbiota
4.1. Host genetic factors, gut microbiota, and multiple sclerosis
Host genetics strongly influence gut microbiome composition, highlighted by twin studies where the gut microbiome of monozygotic twins showed more similarity than dizygotic twins’ microbiome.9,53,54 The importance of host genes on the microbiome was further validated by large genome-wide association studies (mGWAS), which cataloged the association between host gene variants and the gut microbiome.55,56 Among all the genetic factors linked with MS, major histocompatibility complex (MHC) or HLA genes show the strongest association with MS susceptibility. The HLA class-II linkage in MS differs in various populations, but the highest association is with HLA-DR2 (DRB1 × 1501)/DQ6 (DQB1 × 0602),57 followed by DR3/DQ2 and DR4/DQ8 haplotypes.6,55,58 A study involving 3,002 public human gut microbiota datasets showed that individuals with functionally similar HLA haplotypes are also similar in the microbiota composition,59 suggesting a possible linkage between genetics and the microbiome in MS predisposition.
However, there are limited data on the role of HLA class-II restricted gut microbiota in the modulation of disease in MS/EAE. A direct role of HLA class-II genes on gut microbiota was suggested in Myelin Basic Protein (MBP)-specific T-Cell Receptor (TCR) transgenic mice on HLA-DRβ1 × 1501 background, which developed spontaneous EAE.60 Utilizing HLA class-II transgenic mice, we have shown that HLA class-II polymorphism modulates gut microbiota composition, which may be responsible for the difference in disease phenotype between single and double HLA-II transgenic mice in EAE.61,62 Specifically, while HLA-DQ8 mice are resistant to EAE, HLA-DR3.DQ8 mice develop more severe disease than HLA-DR3 mice in an IL-17A-dependent manner. Prior studies have shown that certain gut bacteria can induce IL-17A-secreting CD4+ T cells,63,64 DQ8-restricted gut microbiota may increase disease severity in HLA-DR3.DQ8 mice through the induction of IL-17A. Thus, HLA class-II may affect MS susceptibility by influencing gut microbiota composition and associated cytokine networks.
In addition to the HLA genes, approximately 200 autosomal non-MHC gene variants have been reported as possible risk alleles for MS,8,65 which may modulate gut microbiota composition. However, a direct link between these gene variants and gut microbiota is lacking.
4.2. Dietary factors, gut microbiota, and multiple sclerosis
Among all factors linked with the gut microbiota, the diet has emerged to have the strongest influence on the composition and function of the gut microbiome (Figure 1). As our ancestors consumed diverse plant-based diets, gut microbes co-evolved, providing essential enzymes for digesting complex fibers and unlocking additional food sources.66,67 Adapting to varying environments, this symbiotic relationship transformed humans into holobionts, relying on gut bacteria for functions like vitamin production, nutrient digestion, and immune regulation.68 Since the gut microbiome is so intertwined with human physiology, any disruption to this delicate balance can have far-reaching consequences. Environmental changes, particularly those related to diet, can disrupt the delicate balance of the gut microbiome (dysbiosis), which has been associated with a growing number of diseases, including MS.69 A diet rich in components like fiber, phytoestrogens, tryptophan, fats, and sugars, significantly shapes the composition and function of our gut microbiota (Figure 2), which in turn plays a vital role in our overall health. In this section, we will discuss how these dietary elements can either positively or negatively impact our gut microbiome and, consequently, pathobiology of MS. Additionally, we will discuss how gut bacteria-mediated bile acid metabolism can influence both immune function and neuromodulation (Figure 2).
Figure 2.
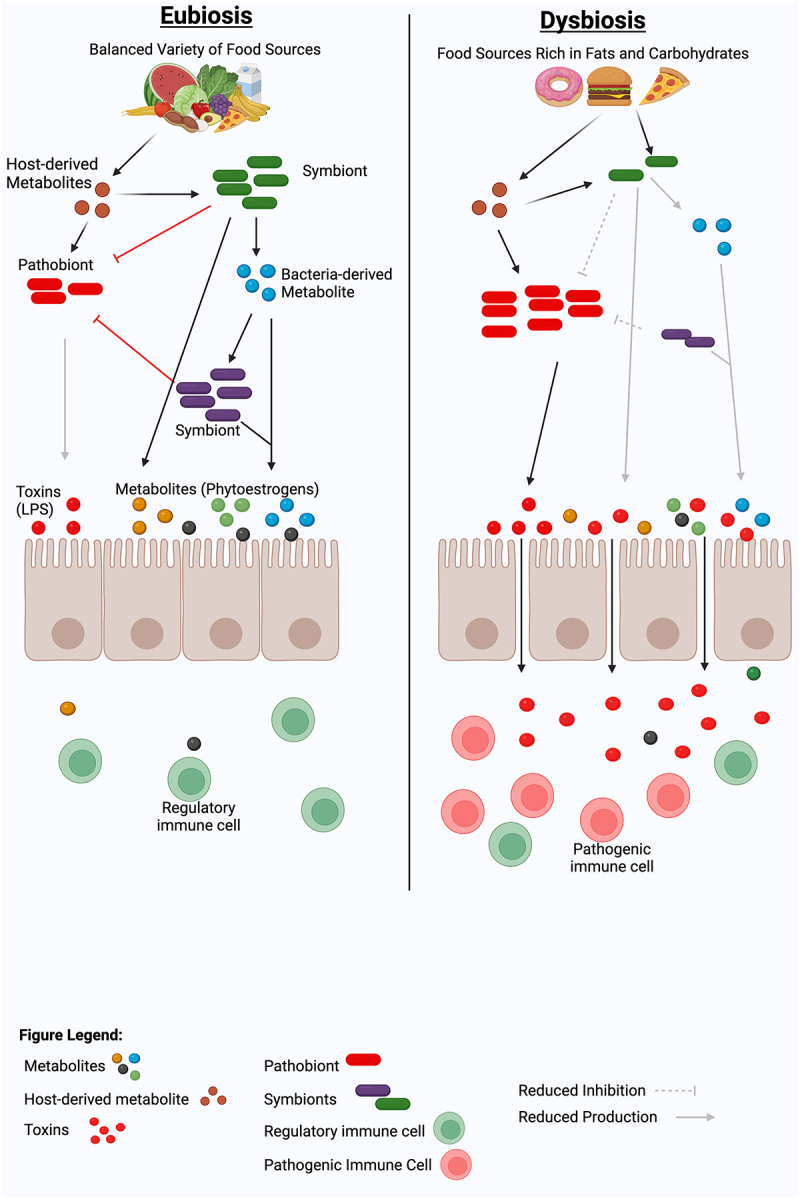
Dietary modulation of gut microbiome and metabolites for immune balance and Eubiosis. A healthy diet, such as a diet rich in fibers, isoflavones, or tryptophan, can promote a diverse, balanced gut microbiota that can maintain a healthy eubiotic state by inducing immunoregulatory cells and cytokines. In contrast, a lack of beneficial plant metabolites in the diet or enrichment of a high-fat or high-fructose diet can induce a dysbiotic gut microbiota characterized by the loss of beneficial gut bacteria and the acquisition of immunostimulatory bacterial molecules such as lipopolysaccharide (LPS). This dysbiotic gut microbiota can predispose or propagate the disease by inducing pathogenic immune cells, which can induce local and/or systemic pro-inflammatory responses. Figure created with BioRender.com.
4.2.1. Fibers
Dietary fiber, the indigestible part of plant foods, is a vital fuel source for the beneficial bacteria residing in our gut.70,71 When gut bacteria ferment these complex carbohydrates, they produce SCFAs, including acetate, propionate, and butyrate. SCFAs play a multifaceted role in maintaining human health. They provide energy for our gut cells, help regulate blood sugar levels, boost our immune system, and reduce inflammation.72 Additionally, SCFAs influence gut barrier function, protecting against harmful pathogens and potentially signaling to our brains, influencing appetite and mood.72
SCFAs are known to provide many beneficial functions to the host both locally and systemically. One of the major functions linked with SCFAs, specifically butyrate and propionate, is their ability to induce Treg generation in the colon and periphery.73,74 SCFAs mediate this effect through G protein-coupled receptors and Histone Deacetylase (HDAC) inhibition, leading to decreased TLR signaling and repression of the NLRP3 inflammasome. As a result, SCFAs can reduce excessive inflammatory responses and promote anti-inflammatory responses, including the generation of Tregs.73 SCFAs are also critical for intestinal barrier stability, regulating the production of IL-10 and IL-22 by activating Signal Transducer and Activator of Transcription 3 (STAT3) and promoting epithelial regeneration and anti-microbial peptide production.75 Overall, SCFAs have potent immunomodulatory activity critical to maintaining gut homeostasis.
SCFAs are mostly absorbed and metabolized by colonocytes, and only a small portion of SCFAs reach systemic circulation. In germ-free (GF) mice, microglia appear stunted, and SCFA supplementation was sufficient to induce their maturation.76 Additionally, antibiotic-induced perturbations to gut microbiota have been shown to influence neuroinflammation and affect microglia.77,78 In vitro, butyrate treatment has shifted microglia to a more anti-inflammatory profile characterized by reduced IL-1β, IL-6, and TNF-α expression.79 SCFAs also regulate the expression of tryptophan 5-hydroxylase, the rate-limiting enzyme in the serotonin production.80 Furthermore, SCFAs, especially butyrate, enter the brain via monocarboxylate transporters (MCTs) found on the blood–brain barrier and central nervous system cells. MCT-1, present in both intestinal and CNS cells, especially oligodendrocytes, astrocytes, and neurons,81 transports butyrate and lactate, acting as an energy source and supporting oligodendrocyte survival. MCT-2, mainly in neurons, exclusively transports butyrate and is involved in synapse repair.
In MS, both total SCFA production and the SCFA profile are altered and characterized by decreases in either acetate, butyrate, or propionate.17,82,83 Strikingly, in one study, propionate supplementation to therapy-naïve PwMS led to a significant increase in functionally competent Tregs and a decrease in Th1 and Th17 cells. Post-hoc analysis also showed that propionate supplementation reduced the annual relapse rate.17 Given that glial cells like microglia, astrocytes, and oligodendrocytes are key players in the pathobiology of MS, the ability of SCFAs to influence the function of these cells, along with neurons, suggests a potential neuroprotective role of SCFA in MS.
In summary, SCFAs may modulate MS disease severity in various ways, including neuro and immune modulation to maintain an anti-inflammatory and neuroprotective phenotype. However, further research is needed to elucidate the precise mechanism through which the neuroactive and immunomodulatory capabilities of SCFAs ameliorate clinical disease.
4.2.2. Phytoestrogens
Phytoestrogens are plant-derived polyphenols with structural similarities to human estrogens and are comprised of several classes of chemical compounds, such as isoflavones (soy) and lignans (flaxseed).84,85 Humans do not have the capacity to metabolize phytoestrogen, but certain gut bacteria can metabolize them to produce metabolites such as S-equol from isoflavones.86 Daidzein and Genistein are the two important isoflavones and are often present as glycosides or aglycones in plant products. These isoflavones are metabolized by gut bacteria belonging to the genera Adlercreutzia,87 Bifidobacterium,88 Eggerthella,89,90 Lactobacillus,91 Slackia,92 etc. to end products S-equol and/or O-DMA depending upon the dietary habits and type of gut bacteria present.93 Importantly, phytoestrogen metabolizing bacteria Prevotella, Parabacteroides, Adlercreutzia, Slackia, and Lactobacillus were lower in PwMS compared to healthy controls.39,42
Phytoestrogens have shown immunomodulatory and neuroprotective effects in multiple studies.85,94 Phytoestrogen and their metabolites can interact with estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ)95 that are expressed in human and mouse immunogenic cells, including T cells, B cells, Natural Killer (NK) cells, macrophages, and dendritic cells (DC).96 S-equol has a strong estrogenic,97 antioxidant,98 and anti-androgenic99 activity. We have summarized the effect of phytoestrogen compounds on these cells previously,85 which indicates that they can exert both local and systemic immunological effects.
While the neuroprotective effects of equol have not been directly studied in MS or EAE, studies in other neuroinflammatory diseases and models suggest its neuroprotective potential.20,21,100 S-equol can reduce neuroinflammation through modulation of the TLR4/NF-kappaB pathway, restoration of neurotransmitter balance, and promotion of synaptic plasticity.20 Additionally, equol has been shown to mediate neuroprotective properties by reducing neuronal death and promoting neurite outgrowth, possibly by enhancing neurotrophin production in astrocytes.20
Phytoestrogens, especially isoflavones, have been shown to exert anti-inflammatory effects in the gut and protect from EAE.101–104 We have shown that the disease-protective effect of isoflavones was dependent on the presence of gut bacteria, especially those with the ability to metabolize dietary isoflavones into S-equol.104 Furthermore, we have shown that dietary isoflavones can modulate the gut microbiota to enrich beneficial bacteria and reduce EAE severity by altering lipopolysaccharides (LPS) biosynthesis.103 In addition, dietary isoflavones have been shown to reduce inflammation by modulating phenylalanine and lipid metabolism.105 Presently, there is a lack of research investigating the connection between phytoestrogen levels in PwMS and disease severity, as well as the potential benefits of phytoestrogen supplementation in MS. Future studies may shed light on the importance of phytoestrogens and their metabolism by gut bacteria in the context of MS.
In conclusion, the complex interplay between dietary phytoestrogens, gut microbiota, and their metabolites highlights the potential for significant influence on immune regulation and neuroprotection. The observed reduction of phytoestrogen-metabolizing bacteria in PwMS suggests a potential link between gut dysbiosis and an impaired ability to generate beneficial metabolites like S-equol. While S-equol’s immunomodulatory effects are of interest, the recent findings about isoflavone-induced alterations in LPS biosynthesis underscore the multifaceted mechanisms through which the gut microbiome mediates the beneficial effects of phytoestrogens. Further research is crucial to fully elucidate these mechanisms, potentially opening doors to novel therapeutic approaches for PwMS, targeting both the gut microbiome and phytoestrogen-derived metabolites.
4.2.3. Tryptophan
L-tryptophan is an essential amino acid found both in meat and plant-based foods and is crucial for various physiological functions.106 It can be absorbed from the gut into the bloodstream, and once in circulation, it is transformed through various metabolic pathways, including the kynurenine and serotonin pathways, contributing to numerous physiological processes.22 Within the gut, tryptophan serves as a substrate for metabolism by resident bacteria, leading to the production of beneficial metabolites.106 Gut microbiota catabolizes tryptophan into tryptamine and various immunomodulatory indole derivatives, including indole-3-aldehyde, indole-3-acetic-acid, and indole-3-propionic acid.107 These indole derivatives exert their functions by activating the aryl hydrocarbon receptor (AhR), leading to many downstream events essential for gut homeostasis.108 Lactobacillus species, bacteria known to catabolize tryptophan, were shown to attenuate gut inflammation via AhR and regulate IL-22 production to protect against fungal infection.109,110 Additionally, Lactobacillus reuteri were found to reprogram intraepithelial CD4+ T cells into immunoregulatory cells via indole derivatives.111 Tryptophan catabolites have also been shown to regulate intestinal barrier integrity. Specifically, IPA was found to protect barrier function in a mouse model of colitis via the pregnane X receptor and reduce intestinal permeability in mice fed a high-fat diet.112,113
Additionally, gut microbiota can also influence serotonin synthesis, a tryptophan derivative, where SCFA production increases the expression of Tryptophan Hydroxylase 1 (TPH1), leading to increased serotonin levels.80,114 Certain gut bacteria have been shown to produce serotonin, including Lactobacillus, Lactococcus, and Streptococcus species.115 Importantly, serotonin can attenuate EAE severity by reducing IFNγ production and T cell proliferation.116,117
Tryptophan can also directly affect the CNS resident cells through the kynurenic acid pathway.22 While in a homeostatic condition, kynurenic acid is neuroprotective; during inflammation, kynurenine, a precursor to neurotoxic metabolites like quinolinic acid, is produced by microglia and macrophages in the CNS. Additionally, the tryptophan-derived indole-containing metabolites can induce inflammatory pathways in microglia and astrocytes through the aryl hydrocarbon receptor (AhR).22
In MS, it has been shown that PwMS have lower urinary levels of kynurenine, a known immunosuppressive tryptophan metabolite, which was also negatively correlated with the Expanded Disability Status Scale (EDSS) score.118 In the CNS, Quintana et al. found that supplementation with tryptophan metabolites activated AhR on astrocytes leading to an increase in IFN-I signaling and attenuating EAE.119 Importantly, AhR agonists, including tryptophan metabolites were also reduced in PwMS.119 Interestingly, high tryptophan diet ameliorated EAE and reduced autoreactive T cell activation and migration. Although, these effects were independent of the AhR.120 However, the protective role of AhR activation in CNS autoimmunity is still controversial as Lactobacillus reuteri supplementation can enhance IL-17 production from CD4+ T cells and exacerbate EAE through Ahr activation.121
Altogether, tryptophan catabolism by the gut microbiota is known to have both local and systemic immunomodulatory as well as neuroprotective effects, with the potential to modulate MS disease severity. However, further research is necessary to understand the significance of host and microbial tryptophan metabolism in MS.
4.2.4. High-fat and high-fructose diet
A high-fat diet (HFD), characterized by an excessive intake of fats, particularly unhealthy saturated and trans fats plays a significant role in the rising global incidence of obesity (affecting 650 million people worldwide, with 100 million in the USA) and contributes to inflammatory diseases, including MS.122,123 Obesity has evolved into a global public health crisis, with approximately 35% of adults in the U.S. classified as obese, as reported by the Centers for Disease Control and Prevention (CDC) in 2023. Numerous studies over the last decade have also elucidated the significant impact of obesity as a risk factor for both the susceptibility and severity of MS.124–126
HFD can significantly impact gut barrier integrity and mucosal immune responses.127–129 HFDs have been shown to disrupt the tight junctions between intestinal epithelial cells, leading to increased intestinal permeability, often referred to as “leaky gut”.128 This increased permeability allows for the translocation of harmful substances, such as bacterial toxins (e.g., lipopolysaccharides) and metabolites, from the gut lumen into the bloodstream, triggering systemic inflammation. Furthermore, HFDs can alter the composition of the gut microbiota, promoting the growth of pro-inflammatory bacteria and suppressing beneficial species.128,129 Our recent study on HFD-induced obesity in mice showed gut dysbiosis with enrichment of Proteobacteria and Desulfovibrionaceae and reduced abundance of crucial bactiera that maintain a healthy gut milieu, such as Lactobacillus, Prevotellaceae, and Muribaculaceae.129 This dysbiosis can further exacerbate mucosal immune responses, leading to chronic low-grade inflammation, which is implicated in autoimmune diseases including MS. Notably, the depletion of dysbiotic gut microbiota in HFD-fed mice ameliorated disease severity, underscoring the pivotal role of gut dysbiosis in exacerbating MS susceptibility and severity in the context of HFD-induced obesity.129 HFD induced obesity in mice has been linked to enhanced microglial activation and an increase in pro-inflammatory Th1 and Th17 cells, key players in EAE.127 Furthermore, obesity has been shown to promote EAE through increased levels of IL-6 and CCL-2, cytokines that facilitate the infiltration of T cells into the central nervous system, exacerbating inflammation.127 HFD have been linked to disruption of the blood–brain barrier (BBB) and increased oxidative stress, both of which are implicated in neurodegenerative diseases.130–132 There are limited studies on the ability of HFD-induced obesity to modulate glial cells and neurons in MS or its animal model. However, based on studies in other models of neurological diseases where HFD-induced obesity has been shown to modulate microglia, astrocytes, oligodendrocytes, and neurons.130–132 This suggests that similar pathways might be activated in MS and EAE. In the context of EAE and MS, a compromised BBB could allow for increased infiltration of immune cells and inflammatory molecules into the central nervous system, exacerbating neuroinflammation.
Besides high fats, a diet rich in sugar, especially high fructose syrup has also emerged as an important factor in obesity.133 Although, there are no studies on the role of sugar intake on MS, a study in patient with neuromyelitis optica spectrum disorder has shown a link between higher sugar intake and disease severity.134 We have analyzed effect of Fructose-rich diet (FRD) in mice and EAE model.135 We have shown that mice on FRD lost beneficial bacteria such as Prevotella, Muribaculum, and Bifidobacterium135 and enriched for Desulfovibrio, Collinsella, Olsenella, and Bacteroides species. Additionally, mice on FRD show enrichment of immune populations linked with pro-inflammatory phenotypes such as Helios-RORγt+FoxP3+CD4+ T cells in the small intestine.135 Surprisingly, despite the changes observed in the gut microbiota and immune system, an FRD had only a minor impact on the severity of EAE. Thus, further study is warranted to determine the precise role of high fructose on the pathobiology of EAE and MS.
In conclusion, the gut microbiome plays a crucial role in how diets high in fat or fructose negatively impact health. Interestingly, certain bacterial taxa, such as Desulfovibrio, Prevotellaceae, and Muribaculaceae, were altered in both the HFD and FRD groups, indicating a possible shared pathway for triggering inflammatory responses. A better understanding of how these diets alter the composition and function of gut bacteria could pave the way for improved treatments for obese PwMS.
4.3. Bile acids
Bile acids (BA) are amphipathic molecules generated in the liver and stored in the gall bladder after cholesterol breakdown.136 There are two mechanisms for BA synthesis. The classical pathway produces primary BA, which conjugates with glycine and taurine in the liver before being stored in the gall bladder.137,138 BA is released from the gall bladder and into the small intestine, which aids digestion and absorption of nutrients.137 If BAs are not absorbed in the intestines, the alternative pathway occurs where gut microbiota converts BAs into secondary BAs. Eventually, the secondary BAs will be absorbed in the colon and later return to the liver for enterohepatic circulation.139
BA composition and levels are important for regulating gut diversity and homeostasis.138 The gut microbiome can deconjugate, dehydroxylate, and dehydrogenate BAs.139 Bacteria, such as Lactobacillus. spp., Bifidobacterium spp., and Enterococcus spp., deconjugate BA (remove glycine and taurine) in the small intestine via bile salt hydrolases.140,141 After deconjugation, some bacteria, like Clostridium spp., can convert cholic acid and chenodeoxycholic acid (CDCA) into deoxycholic acid (DCA) and lithocholic acid (LCA) via dihydroxylation.138 Gut microbes will dehydrogenate and epimerize CDCA to ursodeoxycholic acid while also converting DCA and LCA to iso-DCA and isoLCA.142,143 These processes increase BA solubility, which is necessary for BA functions.144 BAs have broad functions, including interacting with cell surface and nuclear receptors, membranes, the immune system, the nervous system, and the gut microbiome.145,146 Additionally, BA composition can be affected by diet, sex differences, and antibiotic treatment. High- and low-fat diets can decrease primary BA synthesis, while high-protein diets can increase DCA, LCA, and CDCA levels/BA synthesis.147,148 BA composition also varies with age and sex. Decreased BA levels are commonly found in men but not women.149
In recent studies by Bhargava et al., human BA levels were reported to be abnormal in PwMS, and their receptors are present in MS brain lesions.136 In PwMS, gut dysbiosis was associated with the presence or absence of BA-metabolizing bacteria.38,150 PwMS harbor different gut microbiota depending on their stage of disease. Specifically, Chen et al. found BA-metabolizing bacteria, like Parabacteroides and Erysipelotrichaceae, to be decreased in PwMS.39,42 The absence of these bacteria can result in decreased BA metabolism and metabolite production necessary to support homeostasis at mucosal surfaces.151 In EAE, the animal model of MS, neuroinflammation is associated with altered cholesterol metabolism in astrocytes and abnormal circulating BA metabolites.152
In contrast to conjugated primary BA, gut bacteria-derived unconjugated bile acids readily enter the CNS via a simple diffusion.22 Different bile acids have distinct effects on BBB permeability, with unconjugated CDCA and DCA increasing permeability, while both unconjugated and glycine-conjugated ursodeoxycholic acid (UDCA) strengthen the BBB. Recent studies suggest that BA supplementation, particularly with tauroursodeoxycholic acid (TUDCA), can reduce neuroinflammation.136 TUDCA supplementation ameliorated EAE and limited pathogenic inflammatory pathways in glial cells through G protein-coupled bile acid receptor 1 (GPBAR1) signaling.136
In conclusion, gut microbiota-derived bile acids metabolites represent a complex and multifaceted class of molecules with significant roles in neurological diseases like MS. The observed dysbiosis in PwMS, particularly the changes in bile acid-metabolizing bacteria, suggests a crucial interplay between the gut microbiome, bile acid profiles, and neuroinflammation.
5. Mechanisms through which gut dysbiosis promotes inflammation and disease
During a healthy state, the gut is lined with intestinal epithelial cells (IECs) that separate microbes and their products from host cells/tissues due to tight junctional proteins.153 However, dysbiosis may promote a cascade of events, including the enrichment of pathogenic bacteria and the release of deleterious toxins, leading to a pro-inflammatory environment and a compromised gut barrier.154,155 A key player in this process is thought to be pathogen-associated molecular patterns (PAMPs), including LPS, a component of gram-negative bacteria.156 This increased gut permeability (Leaky gut) permits PAMPs such as LPS and other bacterial metabolites to enter the bloodstream, triggering widespread systemic inflammation. Additionally, certain bacteria can directly influence the immune system, shaping the development and behavior of immune cells such as CD4 T cells, B cells, dendritic cells (DCs), and macrophages.157 The combination of LPS-induced inflammation, leaky gut, and immune activation creates a perfect storm for dysregulated immune activation that can fuel chronic diseases. Furthermore, dysbiosis can lead to changes in the metabolites produced by gut bacteria, and a reduction in health-promoting metabolites, like short-chain fatty acids, can contribute to a pro-inflammatory environment.158 This chronic state of inflammation sets the stage for the development of various diseases, including MS. Particularly in the context of MS, bacterial metabolites either can help in overcoming tolerance to autoimmune response and/or can contribute to the propagation of disease as discussed below.
5.1. Lipopolysaccharides
Lipopolysaccharides (LPS) are surface glycolipids found in the outer membrane of most Gram-negative bacteria. Lipid A, the core oligosaccharide, and the O-antigen comprise LPS. LPS is known to stimulate the immune system and produce a strong immune response mediated mostly through Toll-Like Receptors (TLR)-2 and TLR-4 receptors.159,160 The overall structure of LPS is conserved; however, several variations can be found between bacteria in all three structural moieties.161–163 Thus, differences in the bacterial populations in the gut microbiota can directly affect the composition and amount of LPS in the gut, determining the local and systemic immune response. Most proinflammatory LPS are derived from Proteobacteria, which increase oxidative stress and result in the production of pro-inflammatory cytokines. The binding of LPS to the LPS-binding protein (an acute phase protein) allows the complex to interact with CD14. This complex of LPS-LPS binding protein and CD14 interacts with TLR4/Myeloid differentiation factor 2 (MD-2) at the cell surface, which subsequently activates the cell through the NF-kB signaling pathway, resulting in the secretion of proinflammatory cytokines such as IFN-y, TNF-α, IL-1β, and IL-8.164 LPS can directly damage the epithelial barrier locally by inducing the expression of inflammatory cytokines like IL-8.165 However, microbiome LPS immunogenicity varies in humans166 and such differential immunological responses can be the result of structural differences of LPS.167
The pro- and anti-inflammatory nature of LPS has been described in multiple inflammatory conditions, including autoimmune diseases. LPS isolated from gut bacteria, especially Bacteroides vulgatus has shown to induce pro-inflammatory endotoxin tolerance acting through MD-2/TLR4 receptor complex in CD11c+ cells of intestinal lamina propria.168 Especially in autoimmune diseases, a human study of 222 infants in Northern Europe has shown that LPS isolated from Bacteroides, and Escherichia coli are structurally different and contribute to the development of autoimmune diabetes differently in mice.166 In MS cases, LPS and LPS binding proteins were observed to be higher in the blood.169 The same study found these two to be higher in the brain, spinal cord, and blood of rats. Mice exposed to LPS at an early age have also been shown to reduce the severity of MOG-induced EAE at 12 weeks, where authors observed increased IL-10 and FOXP3 transcript levels in the spinal cord.170 In addition, we have shown that the isoflavone diet can modulate the composition of gut microbiota and the immunogenicity of LPS.103 Specifically, fecal LPS extracts isolated from the gut microbiota of mice fed with an isoflavone diet-induced anti-inflammatory effects by enhancing IL-10 and reducing IL-12/23 in the EAE.103 In contrast, fecal LPS from mice on a phytoestrogen-free diet-induced proinflammatory cytokines such as IL-1β, IL-6, TNF-α and IL-12.103
Taken together, gut dysbiosis can alter the microbial composition, resulting in either pro- or anti-inflammatory immunological effects based on differences in LPS immunogenicity, consequently affecting the exacerbation or prevention of MS/EAE.
5.2. Leaky gut syndrome
Gut dysbiosis can disrupt the homeostasis at mucosal surfaces due to changes in the composition of the gut microbiota.154 Specifically, when pathobionts are enriched, and symbionts are depleted, gut barrier dysfunction, or “leaky” gut syndrome (LGS), occurs.155 LGS can further be characterized by increased intestinal permeability, allowing for bacterial translocation to occur and the growth and colonization of pathogenic bacteria (pathobionts). When the gut barrier is disrupted, hosts are predisposed to gut-specific as well as systemic and organ-specific diseases including MS.10
LGS is commonly characterized by gut-specific inflammation that can manifest diseases like Crohn’s, celiac disease, and colitis.155 However, LGS is also observed in patients with neurological diseases such as schizophrenia and autism.171,172 Although not completely understood, it is hypothesized that gut dysbiosis can predispose patients to neurological diseases like MS.173–176 Besides gut dysbiosis, PwMS have high levels of pro-inflammatory cytokines, like IL-1β, TNF-α, and IL-6, in their sera and increased gut permeability.177–179 It is further hypothesized that gut dysbiosis can enhance the activation of the immune system to cause more severe demyelination in the CNS.173 Our group and others complement these findings in mice as mice with EAE disease were accompanied by increased gut permeability.180,181 Thus, LGS is linked to MS and can play an important role in the pathobiology of disease.
5.3. Modulation of regulatory T cell by microbiota
Regulatory T cells (Tregs) play a critical role in suppressing effector T cell responses to self-antigens, thus preventing spontaneous autoimmune diseases. Notably, disturbances in the function of CD4+ Tregs have been observed in PwMS, with several studies reporting a significant decrease in suppressive ability.182,183 Additionally, CD8+ Treg function is deficient during acute exacerbation of MS.16,184 Thus, increasing Tregs in PwMS or improving Treg functionality remains an enticing potential therapeutic.
Tregs consist of two distinct populations. The first population develops in the thymus, where thymus-derived Treg (tTreg) cells are generated following recognition of self-antigen by the T cell receptor. The second population, peripherally derived Tregs (pTreg), are stimulated in the periphery, where under certain conditions naïve CD4+ T cells gain expression of FOXP3 upon recognition of their cognate antigen.185 Importantly, pTregs are thought to be dependent on microbiota for expansion and maintenance, whereas gut microbiota composition can dictate pTreg number and function.185 Thus, the Treg pool, specifically pTregs, represents a dynamic population that environmental factors may externally modulate.
Evidence for gut microbiota-dependent modulation of Tregs is highlighted by seminal studies showing colonic Treg induction by SCFA-producing bacteria.186 SCFA supplementation also increased Treg differentiation and ameliorated EAE, while long-chain fatty acids (LCFAs) exerted the opposite effect.187 Additionally, microbiota-induced Treg expansion has been observed in the periphery. Kasper et al. showed that gut commensal Bacteroides fragilis enhanced Treg numbers in cervical lymph nodes and, importantly, ameliorated EAE.188 Dietary intervention resulting in altered gut microbiota composition has also been shown to modulate Tregs and ameliorate CNS autoimmunity. Piccio et al. observed that intermittent fasting ameliorated EAE and increased Treg frequency in gut-associated lymphoid tissue.189 Alterations in gut microbiota composition were highlighted by enrichment of Bacteroidaceae, Lactobacillaceae, and Prevotellaceae families.189 However, changes in gut microbiota composition may also indirectly affect the ability of Tregs to restrain autoimmunity through the induction of Th1 and Th17 cells or through modulation of the T cell microenvironment.190 For example, we have previously shown that an isoflavone-free diet exacerbates EAE and increases the number of IFNγ and IL-17-producing cells in the CNS. However, Treg frequency and number were not altered.104 Further research on the role of microbially induced or expanded Tregs in autoimmunity, as well as a more detailed characterization of Tregs in autoimmune patients, will be necessary to evaluate the ability of microbiome-based therapeutics to modulate the Treg compartment in PwMS.
5.4. Pro-inflammatory T cell induction by microbiota
MS is thought to be mediated by self-reactive, myelin-specific CD4+ T helper cells, with Th17 cells being the most implicated lineage.191 Th17 cells are characterized by their production of the pro-inflammatory cytokine IL-17 and migrate to the CNS during active disease.192,193 However, canonically, Th17s protect mucosal barriers and maintain tolerance toward commensal bacterial flora, making them crucial for intestinal homeostasis.63 Thus, it is hypothesized that dysregulation of Th17 differentiation and expansion of Th17 cells, mediated by gut microbiota, may be involved in the initiation or progression of disease.194 In support of this hypothesis, germ-free mice are resistant to EAE and lack Th17 cells.18 However, mono-colonization with segmented filamentous bacteria (SFB) was sufficient to restore susceptibility to EAE disease and led to the expansion of Th17s in the CNS.18 These data describe a requirement for gut bacteria in EAE pathology and suggest that immunostimulatory bacteria that expand Th17 cell populations may influence clinical disease. Furthermore, Duc. et al. have shown that disrupting myelin-specific Th17 cell trafficking to the colon during EAE significantly attenuates disease, suggesting a role of gut microbiota in catalyzing the encephalitogenic properties of Th17 cells.195 Interestingly, bacterial species that can promote Th17 induction and pro-inflammatory processes have been associated with MS, including Akkermansia muciniphila and Acinetobacter calcoaceticus.38 Akkermansia muciniphila colonization has also exacerbated EAE in vivo.104 These immunostimulatory bacteria may directly induce Th17 cell responses or indirectly through metabolite production. In one study, Lactobacillus reuteri tryptophan metabolism promoted CNS autoimmunity through aryl hydrocarbon receptor (AhR) stimulation leading to increased IL-17 production.121 Understanding how gut bacteria exacerbate clinical disease through the induction of pathogenic Th17 cell populations and the amplification of pro-inflammatory cytokine production or through the expansion of autoreactive Th17s will be critical.
5.5. Regulatory and pro-inflammatory B cells induction by microbiota
B cells have emerged as an important immune cell in the pathogenesis of MS. Specifically, abnormalities have been observed in the quantity and quality of immunoglobulins in the cerebrospinal fluid (CSF) where greater than 90% of patients with MS are positive for Immunoglobulin G (IgG) oligoclonal bands in the CSF.196 B cells also represent a subset of infiltrating cells in the brain and spinal cord of PwMS.197 The significance of these abnormalities is emphasized by the efficacy of B cell depletion therapies in PwMS and the compelling evidence linking Epstein–Barr virus (EBV) infection and anti-EBNA antibody levels to the onset of clinical symptoms.198,199 However, the role of gut microbiota on pathogenic B cell responses in EBV or MS is poorly understood and requires further study.
A gut microbiota-dependent, anti-inflammatory role of B cells in MS has been elucidated. Rojas et al. found that IgA+ plasma cells (PCs) are significantly reduced in the gut during EAE and, importantly, the removal of plasmablasts and PCs resulted in exacerbated EAE.200 A follow-up study found that IgA+ B cells traffic across the blood–brain barrier during active MS and have specificity toward MS-associated immunostimulatory bacterial strains. However, these IgA+ B cells were potent IL-10 producers and do not cross-react with the self-antigen.201 Interestingly, IgA production also appears to be altered in the gut of PwMS, where an increased EDSS score is associated with a decrease in gut IgA-coated bacteria.202
These data highlight the complex role B cells play in the pathobiology of MS. Microbially induced IgA+ B cells may represent a regulatory subset in the CNS, and B cells of other specificities, such as EBV-specific B cells, may exacerbate disease through chronic inflammatory cytokine production and self-antigen cross-reactivity.
6. Microbiome based therapeutics
The gut microbiome is known to influence the pathogenesis of many human diseases, and there is significant evidence supporting gut microbiota alterations in PwMS.10 Thus, targeted interventions to modulate gut microbiota toward a “healthier” composition remain an attractive potential therapy. A variety of approaches are used to modulate gut microbiota, including diet, probiotics, synbiotics, and fecal microbiome transplantation (FMT), all with potential in MS. Multiple dietary regiments have been explored in MS to modify gut microbiome composition and downregulate systemic inflammation.203 The Swank diet, a low-fat diet developed by Dr R. L. Swank, restricts fat consumption to less than 20 g per day in an attempt to reduce cholesterol and improve cerebrovascular health. Importantly, Dr Swank performed a 34-year follow-up study showing that PwMS who consumed less than 20 g of fat were less likely to experience severe exacerbations or death.204 However, Swanks’ studies were not randomized controlled trials and may be biased for a multitude of reasons. The Wahls diet, a modified paleolithic diet developed by Dr Terry Wahls, has also been shown to improve MS symptoms. In a small, randomized waitlist-controlled trial, PwMS exhibited improved fatigue and QOL scores after 3 months on the diet.205 The diet also has the potential to exacerbate the disease. For example, obesity significantly increases the risk and severity of MS, and a high-fat diet, linked with increased incidence of obesity, was shown to induce gut dysbiosis and exacerbate EAE in mice.39,129 However, further study is necessary to understand how diet quality impacts the development and severity of MS symptoms. Probiotic-based therapies have also been used to treat PwMS, and probiotics can ameliorate EAE via multiple mechanisms.117,180 Interestingly, Prevotella histicola supplementation was shown to be as potent as COPAXONE in ameliorating EAE and reduced demyelination and inflammation in the brain.206 However, clinical data on the beneficial effect of probiotics in PwMS has been inconclusive. A meta-analysis of three randomized controlled trials was performed to demonstrate differences between PwMS receiving probiotic or placebo supplementation. While improvements were seen in levels of inflammatory and oxidative stress markers, differences in EDSS and Beck Depression Inventory (BDI) scores were heterogeneous.207
However, in another meta-analysis, probiotics were shown to significantly improve depression and anxiety in PwMS.208 More randomized controlled trials and better approaches to identify immunomodulatory bacteria in PwMS with the potential to modulate disease are necessary. A combination of dietary alterations and probiotic supplementation may also be important to promote the growth of beneficial gut bacteria, commonly known as synbiotic therapy. This approach has shown promise in animal models of MS where Parabacteroides distasonis and Aldercrutzia equolifaciens supplementation in conjunction with an isoflavone-rich diet ameliorated EAE. In contrast, Escherichia coli supplementation and isoflavone diet did not ameliorate EAE.104 FMT is another exciting potential therapy that has shown promise in GI diseases such as Clostridium difficile infection. However, defining what constitutes a “healthy” microbiome with the potential to suppress inflammation and MS remains a challenge.
7. Conclusions
The significance of gut microbiota in the pathobiology of MS is increasingly recognized, presenting a vast potential for leveraging its capabilities as a potential diagnostic, prognostic, and therapeutic tool. However, further investigation is warranted to delineate specific bacteria or bacterial communities associated with the disease, both positively and negatively, as this understanding will be pivotal in harnessing their potential effectively. While the gut microbiome has garnered significant attention in the context of MS, it is crucial to acknowledge that other host microbiomes, such as the oral209–211 and nasal microbiota,211 may also play a role in the development and progression of this neurological disorder. Emerging research suggests that alterations in the composition and function of these microbial communities could influence immune responses and potentially contribute to neuroinflammation.212 Investigating the interplay between these diverse microbiomes and the host immune system represents a promising avenue for future research, potentially uncovering novel insights into MS pathogenesis and paving the way for innovative therapeutic interventions targeting these microbial ecosystems. Additionally, prospective investigations into elucidating the interplay among various gut bacteria affected in PwMS will aid in delineating a disease-modifying gut microbiome. Such studies will also contribute to determining whether a combination of diet and microbiota (synbiotics) may present a more effective treatment strategy for PwMS compared to solely relying on bacteria (probiotics) or diet (prebiotics).
Funding Statement
We want to apologize to all authors whose work cannot be cited due to space constraints. This work was supported by the National Institute of Allergy and Infectious Diseases Grant 1RO1AI137075 (to A.K.M.), Veteran Affairs Merit Award 1I01CX002212 and I01BX006112 (to A.K.M.), University of Iowa Environmental Health Sciences Research Center (EHSRC), National Institute on Environmental Health Sciences/National Institutes of Health Grant P30 ES005605 (to A.K.M.), a gift from P. Heppelmann and M. Wacek (A.K.M.) and the University of Iowa Fraternal Order of Eagles Diabetes Research Center pilot grant (A.K.M.). S.G. is supported by the NIH Institutional Training Grant T32AI007260 (PIs: Butler and Wu). T.T is supported by the NIH Institutional Training Grant T32AI007485 (PIs: Legge). P.L. is supported by a University of Iowa Graduate College fellowship through EHSRC
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Dendrou CA, Fugger L, Friese MA.. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–25. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 2.Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, et al. Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult Scler. 2020;26(14):1816–1821. doi: 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klineova S, Lublin FD. Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(9):a028928. doi: 10.1101/cshperspect.a028928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodin DS, Scalas E. The causal cascade to multiple sclerosis: a model for MS pathogenesis. PLOS ONE. 2009;4(2):e4565. doi: 10.1371/journal.pone.0004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waubant E, Lucas R, Mowry E, Graves J, Olsson T, Alfredsson L, Langer‐Gould A. Environmental and genetic risk factors for MS: an integrated review. Ann Clin Transl Neurol. 2019;6(9):1905–1922. doi: 10.1002/acn3.50862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zivadinov R, Uxa L, Zacchi T, Nasuelli D, Ukmar M, Furlan C, Pozzi-Mucelli R, Tommasi MA, Locatelli L, Ulivi S, Bratina A. HLA-DRB1*1501, -DQB1*0301, -DQB1*0302, -DQB1*0602, and -DQB1*0603 alleles are associated with more severe disease outcome on MRI in patients with multiple sclerosis. Int Rev Neurobiol. 2007;79:521–535. [DOI] [PubMed] [Google Scholar]
- 7.Dyment DA, Herrera BM, Cader MZ, Willer CJ, Lincoln MR, Sadovnick AD, Risch N, Ebers GC. Complex interactions among MHC haplotypes in multiple sclerosis: susceptibility and resistance. Hum Mol Genet. 2005;14(14):2019–2026. doi: 10.1093/hmg/ddi206. [DOI] [PubMed] [Google Scholar]
- 8.Patsopoulos N, editor. 200 loci complete the genetic puzzle of multiple sclerosis. In: American society of human genetics (ASHG) 2016 annual meeting. 2016; Rockville, MD: American Society of Human Genetics, Inc. [Google Scholar]
- 9.Ebers GC, Bulman DE, Sadovnick AD, Paty DW, Warren S, Hader W, Murray TJ, Seland TP, Duquette P, Grey T, et al. A population-based study of multiple sclerosis in twins. N Engl J Med. 1986;315(26):1638–1642. doi: 10.1056/NEJM198612253152603. [DOI] [PubMed] [Google Scholar]
- 10.Mangalam AK, Yadav M, Yadav R. The emerging world of microbiome in autoimmune disorders: opportunities and challenges. Indian J Rheumatol. 2021;16(1):57–72. doi: 10.4103/injr.injr_210_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez Murua S, Farez MF, Quintana FJ. The immune response in multiple sclerosis. Annu Rev Pathol Mech Dis. 2022;17(1):121–139. doi: 10.1146/annurev-pathol-052920-040318. [DOI] [PubMed] [Google Scholar]
- 12.Sospedra M, Martin R. Immunology of multiple sclerosis. Semin Neurol. 2016;36(2):115–127. doi: 10.1055/s-0036-1579739. [DOI] [PubMed] [Google Scholar]
- 13.Laschinger M, Engelhardt B. Interaction of alpha 4-integrin with VCAM-1 is involved in adhesion of encephalitogenic T cell blasts to brain endothelium but not in their transendothelial migration in vitro. J Neuroimmunol. 2000;102(1):32–43. doi: 10.1016/S0165-5728(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 14.McKenna PG, Yasseen AA. Increased sensitivity to cell killing and mutagenesis by chemical mutagens in thymidine-kinase-deficient subclones of a friend murine leukaemia cell line. Genet Res. 1982;40(2):207–212. doi: 10.1017/S0016672300019078. [DOI] [PubMed] [Google Scholar]
- 15.Venken K, Hellings N, Liblau R, Stinissen P. Disturbed regulatory T cell homeostasis in multiple sclerosis. Trends Mol Med. 2010;16(2):58–68. doi: 10.1016/j.molmed.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Sinha S, Boyden AW, Itani FR, Crawford MP, Karandikar NJ. CD8(+) T-Cells as immune regulators of multiple sclerosis. Front Immunol. 2015;6:619. doi: 10.3389/fimmu.2015.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, Bader V, Haase S, Kaisler J, David C, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180(6):1067–80 e16. doi: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen T, Noto D, Hoshino Y, Mizuno M, Miyake S. Butyrate suppresses demyelination and enhances remyelination. J Neuroinflammation. 2019;16(1):165. doi: 10.1186/s12974-019-1552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu C, Gao R, Zhang Y, Jiang N, Chen Y, Sun J, Wang Q, Fan B, Liu X, Wang F, et al. S-equol, a metabolite of dietary soy isoflavones, alleviates lipopolysaccharide-induced depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity. Food Funct. 2021;12(13):5770–5778. doi: 10.1039/D1FO00547B. [DOI] [PubMed] [Google Scholar]
- 21.Subedi L, Ji E, Shin D, Jin J, Yeo JH, Kim SY. Equol, a dietary Daidzein Gut metabolite attenuates microglial activation and potentiates neuroprotection in vitro. Nutrients. 2017;9(3):207. doi: 10.3390/nu9030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fettig NM, Osborne LC. Direct and indirect effects of microbiota-derived metabolites on neuroinflammation in multiple sclerosis. Microbes Infect. 2021;23(6–7):104814. doi: 10.1016/j.micinf.2021.104814. [DOI] [PubMed] [Google Scholar]
- 23.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab. 2015;26(9):493–501. doi: 10.1016/j.tem.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31(1):69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson AS, Koller KR, Ramaboli MC, Nesengani LT, Ocvirk S, Chen C, Flanagan CA, Sapp FR, Merritt ZT, Bhatti F, et al. Diet and the human gut microbiome: an international review. Digestive Dis Sci. 2020;65(3):723–740. doi: 10.1007/s10620-020-06112-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16(7):1024–1033. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng ZH, Zhu Y, Li QL, Zhao C, Zhou PH. Enteric nervous system: the bridge between the gut microbiota and neurological disorders. Front Aging Neurosci. 2022;14:810483. doi: 10.3389/fnagi.2022.810483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer EA, Nance K, Chen S. The gut–brain axis. Annu Rev Med. 2022;73(1):439–453. doi: 10.1146/annurev-med-042320-014032. [DOI] [PubMed] [Google Scholar]
- 31.Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and Therapeutic relevance of Neuro-immune communication. Immunity. 2017;46(6):927–942. doi: 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bicknell B, Liebert A, Borody T, Herkes G, McLachlan C, Kiat H. Neurodegenerative and neurodevelopmental diseases and the Gut-brain Axis: the potential of therapeutic targeting of the microbiome. IJMS. 2023;24(11):9577. doi: 10.3390/ijms24119577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakakibara R. Gastrointestinal dysfunction in neuroinflammatory diseases: multiple sclerosis, neuromyelitis optica, acute autonomic ganglionopathy and related conditions. Auton Neurosci. 2021;232:102795. doi: 10.1016/j.autneu.2021.102795. [DOI] [PubMed] [Google Scholar]
- 34.Sakakibara R. Gastrointestinal dysfunction in multiple sclerosis and related conditions. Semin Neurol. 2023;43(4):598–608. doi: 10.1055/s-0043-1771462. [DOI] [PubMed] [Google Scholar]
- 35.Turner T-A, Mangalam AK. The role of the gut microbiome in neurological diseases. Reference module in neuroscience and biobehavioral psychology. Elsevier; 2024. [Google Scholar]
- 36.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci USA. 2017;114(40):10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cantoni C, Lin Q, Dorsett Y, Ghezzi L, Liu Z, Pan Y, Chen K, Han Y, Li Z, Xiao H, et al. Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine. 2022;76:103798. doi: 10.1016/j.ebiom.2021.103798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA. 2017;114(40):10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, Luckey DH, Marietta EV, Jeraldo PR, Chen X, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6(1):28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, Radice E, Mariani A, Testoni PA, Canducci F, et al. High frequency of intestinal T H 17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. 2017;3(7):e1700492. doi: 10.1126/sciadv.1700492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Baumann R, Gao X, Mendoza M, Singh A, Xia Z, Cox LM, Chitnis T, Yoon H, Moles L, et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. 2022;185(19):3467–86 e16. doi: 10.1016/j.cell.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7(1):12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim S-W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One. 2015;10(9):e0137429. doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thirion F, Sellebjerg F, Fan Y, Lyu L, Hansen TH, Pons N, Levenez F, Quinquis B, Stankevic E, Søndergaard HB, et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. 2023;15(1):1. doi: 10.1186/s13073-022-01148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tremlett H, Fadrosh DW, Faruqi AA, Hart J, Roalstad S, Graves J, Lynch S, Waubant E, Aaen G, Belman A, et al. Gut microbiota composition and relapse risk in pediatric MS: a pilot study. J Neurol Sci. 2016;363:153–157. doi: 10.1016/j.jns.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav M, Ali S, Shrode RL, Shahi SK, Jensen SN, Hoang J, Cassidy S, Olalde H, Guseva N, Paullus M, et al. Multiple sclerosis patients have an altered gut mycobiome and increased fungal to bacterial richness. PLoS One. 2022;17(4):e0264556. doi: 10.1371/journal.pone.0264556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirza AI, Zhu F, Knox N, Forbes JD, Bonner C, Van Domselaar G, Bernstein CN, Graham M, Marrie RA, Hart J, et al. The metabolic potential of the paediatric-onset multiple sclerosis gut microbiome. Mult Scler Relat Disord. 2022;63:103829. doi: 10.1016/j.msard.2022.103829. [DOI] [PubMed] [Google Scholar]
- 48.Castillo-Alvarez F, Perez-Matute P, Oteo JA, Marzo-Sola ME. The influence of interferon beta-1b on gut microbiota composition in patients with multiple sclerosis. Neurologia. 2021;36(7):495–503. doi: 10.1016/j.nrleng.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Shah S, Locca A, Dorsett Y, Cantoni C, Ghezzi L, Lin Q, Bokoliya S, Panier H, Suther C, Gormley M, et al. Alterations of the gut mycobiome in patients with MS. EBioMedicine. 2021;71:103557. doi: 10.1016/j.ebiom.2021.103557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochoa-Reparaz J, Kirby TO, Kasper LH. The gut microbiome and multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(6):a029017. doi: 10.1101/cshperspect.a029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasko NJ, Nichols F, Clark RB. Multiple sclerosis, the microbiome, TLR2, and the hygiene hypothesis. Autoimmun Rev. 2020;19(1):102430. doi: 10.1016/j.autrev.2019.102430. [DOI] [PubMed] [Google Scholar]
- 52.Horton MK, McCauley K, Fadrosh D, Fujimura K, Graves J, Ness J, Wheeler Y, Gorman MP, Benson LA, Weinstock‐Guttman B, et al. Gut microbiome is associated with multiple sclerosis activity in children. Ann Clin Transl Neurol. 2021;8(9):1867–1883. doi: 10.1002/acn3.51441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18(11):690–699. doi: 10.1038/nrg.2017.63. [DOI] [PubMed] [Google Scholar]
- 54.Bubier JA, Chesler EJ, Weinstock GM. Host genetic control of gut microbiome composition. Mamm Genome. 2021;32(4):263–281. doi: 10.1007/s00335-021-09884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinshenker BG, Santrach P, Bissonet AS, McDonnell SK, Schaid D, Moore SB, Rodriguez M. Major histocompatibility complex class II alleles and the course and outcome of MS: a population-based study. Neurology. 1998;51(3):742–747. doi: 10.1212/WNL.51.3.742. [DOI] [PubMed] [Google Scholar]
- 56.Cotsapas C, Mitrovic M. Genome-wide association studies of multiple sclerosis. Clin & Trans Imm. 2018;7(6):e1018. doi: 10.1002/cti2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barcellos LF, Sawcer S, Ramsay PP, Baranzini SE, Thomson G, Briggs F, Cree BCA, Begovich AB, Villoslada P, Montalban X, et al. Heterogeneity at the HLA-DRB1 locus and risk for multiple sclerosis. Hum Mol Genet. 2006;15(18):2813–2824. doi: 10.1093/hmg/ddl223. [DOI] [PubMed] [Google Scholar]
- 58.Marrosu MG, Murru MR, Costa G, Cucca F, Sotgiu S, Rosati G, Muntoni F. Multiple sclerosis in Sardinia is associated and in linkage disequilibrium with HLA-DR3 and -DR4 alleles. Am J Hum Genet. 1997;61(2):454–457. doi: 10.1016/S0002-9297(07)64074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andeweg SP, Kesmir C, Dutilh BE, Round J. Quantifying the impact of human leukocyte antigen on the human gut microbiota. mSphere. 2021;6(4):e0047621. doi: 10.1128/mSphere.00476-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yadav SK, Boppana S, Ito N, Mindur JE, Mathay MT, Patel A, Dhib-Jalbut S, Ito K. Gut dysbiosis breaks immunological tolerance toward the central nervous system during young adulthood. Proc Natl Acad Sci USA. 2017;114(44):E9318–E27. doi: 10.1073/pnas.1615715114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mangalam AK, Taneja V, David CS. HLA class II molecules influence susceptibility versus protection in inflammatory diseases by determining the cytokine profile. J Immunol. 2013;190(2):513–518. doi: 10.4049/jimmunol.1201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mangalam A, Luckey D, Basal E, Behrens M, Rodriguez M, David C. HLA-DQ6 (DQB1*0601)-restricted T cells protect against experimental autoimmune encephalomyelitis in HLA-DR3.DQ6 double-transgenic mice by generating anti-inflammatory ifn-gamma. J Immunol. 2008;180(11):7747–7756. doi: 10.4049/jimmunol.180.11.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivanov AK II, Manel N, Brodie EL, Shima T, Karaoz U, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivanov II, Littman DR. Segmented filamentous bacteria take the stage. Mucosal Immunol. 2010;3(3):209–212. doi: 10.1038/mi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shirai R, Yamauchi J. New insights into risk genes and their candidates in multiple sclerosis. Neurol Int. 2022;15(1):24–39. doi: 10.3390/neurolint15010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ehlers S, Kaufmann SH. Participants of the 99 dahlem C. Infection, inflammation, and chronic diseases: consequences of a modern lifestyle. Trends Immunol. 2010;31(5):184–190. doi: 10.1016/j.it.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 68.van de Guchte M, Blottière HM, Doré J. Humans as holobionts: implications for prevention and therapy. Microbiome. 2018;6(1):81. doi: 10.1186/s40168-018-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang GG, Trevaskis NL, Murphy AJ, Febbraio MA. Diet-induced gut dysbiosis and inflammation: key drivers of obesity-driven NASH. Science. 2023;26(1):105905. doi: 10.1016/j.isci.2022.105905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585):212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41(3):283–297. doi: 10.1007/s00281-019-00733-8. [DOI] [PubMed] [Google Scholar]
- 72.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-Brain communication. Front Endocrinol. 2020;11:11. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, deRoos P, Liu H, Cross JR, Pfeffer K, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, Xiao Y, Huang X, Eaves-Pyles TD, Golovko G, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11(3):752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jang HM, Lee HJ, Jang SE, Han MJ, Kim DH. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018;11(5):1386–1397. doi: 10.1038/s41385-018-0042-3. [DOI] [PubMed] [Google Scholar]
- 78.Minter MR, Hinterleitner R, Meisel M, Zhang C, Leone V, Zhang X, Oyler-Castrillo P, Zhang X, Musch MW, Shen X, et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1ΔE9 murine model of Alzheimer’s disease. Sci Rep. 2017;7(1):10411. doi: 10.1038/s41598-017-11047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soliman ML, Puig KL, Combs CK, Rosenberger TA. Acetate reduces microglia inflammatory signaling in vitro. J Neurochem. 2012;123(4):555–567. doi: 10.1111/j.1471-4159.2012.07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. Faseb J. 2015;29(4):1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barcutean L, Maier S, Burai-Patrascu M, Farczadi L, Balasa R. The immunomodulatory potential of short-chain fatty acids in multiple sclerosis. Int J Mol Sci. 2024;25(6):3198. doi: 10.3390/ijms25063198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moles L, Delgado S, Gorostidi-Aicua M, Sepulveda L, Alberro A, Iparraguirre L, Suárez JA, Romarate L, Arruti M, Muñoz-Culla M, et al. Microbial dysbiosis and lack of SCFA production in a Spanish cohort of patients with multiple sclerosis. Front Immunol. 2022;13:960761. doi: 10.3389/fimmu.2022.960761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olsson A, Gustavsen S, Nguyen TD, Nyman M, Langkilde AR, Hansen TH, Sellebjerg F, Oturai AB, Bach Søndergaard H. Serum short-chain fatty acids and associations with inflammation in newly diagnosed patients with multiple sclerosis and healthy controls. Front Immunol. 2021;12:661493. doi: 10.3389/fimmu.2021.661493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Branca F, Lorenzetti S. Health effects of phytoestrogens. Forum Nutr. 2005;(57):100–111. doi: 10.1159/000083773. PMID: 15702593. [DOI] [PubMed] [Google Scholar]
- 85.Cady N, Peterson SR, Freedman SN, Mangalam AK. Beyond metabolism: the complex interplay between dietary phytoestrogens, gut bacteria, and cells of nervous and immune systems. Front Neurol. 2020;11:150. doi: 10.3389/fneur.2020.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sirotkin AV, Harrath AH. Phytoestrogens and their effects. Eur J Pharmacol. 2014;741:230–236. doi: 10.1016/j.ejphar.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 87.Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. Adlercreutzia equolifaciens gen. nov. sp. nov. an equol-producing bacterium isolated from human faeces, and emended description of the genus eggerthella. Int J Syst Evol Microbiol. 2008;58(Pt 5):1221–1227. doi: 10.1099/ijs.0.65404-0. [DOI] [PubMed] [Google Scholar]
- 88.Mustafa SE, Mustafa S, Abas F, Manap M, Ismail A, Amid M, Elzen S. Optimization of culture conditions of soymilk for equol production by Bifidobacterium breve 15700 and Bifidobacterium longum BB536. Food Chem. 2019;278:767–772. doi: 10.1016/j.foodchem.2018.11.107. [DOI] [PubMed] [Google Scholar]
- 89.Yokoyama S, Suzuki T. Isolation and characterization of a novel equol-producing bacterium from human feces. Biosci Biotechnol Biochem. 2008;72(10):2660–2666. doi: 10.1271/bbb.80329. [DOI] [PubMed] [Google Scholar]
- 90.Wang XL, Hur HG, Lee JH, Kim KT, Kim SI. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl Environ Microbiol. 2005;71(1):214–219. doi: 10.1128/AEM.71.1.214-219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heng Y, Kim MJ, Yang HJ, Kang S, Park S. Lactobacillus intestinalis efficiently produces equol from daidzein and chungkookjang, short-term fermented soybeans. Arch Microbiol. 2019;201(8):1009–1017. doi: 10.1007/s00203-019-01665-5. [DOI] [PubMed] [Google Scholar]
- 92.Jin JS, Kitahara M, Sakamoto M, Hattori M, Benno Y. Slackia equolifaciens sp. nov. a human intestinal bacterium capable of producing equol. Int J Syst Evol Microbiol. 2010;60(Pt 8):1721–1724. doi: 10.1099/ijs.0.016774-0. [DOI] [PubMed] [Google Scholar]
- 93.Bolca S, Possemiers S, Herregat A, Huybrechts I, Heyerick A, De Vriese S, Verbruggen M, Depypere H, De Keukeleire D, Bracke M, et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr. 2007;137(10):2242–2246. doi: 10.1093/jn/137.10.2242. [DOI] [PubMed] [Google Scholar]
- 94.Chen HQ, Wang XJ, Jin ZY, Xu XM, Zhao JW, Xie ZJ. Protective effect of isoflavones from Trifolium pratense on dopaminergic neurons. Neurosci Res. 2008;62(2):123–130. doi: 10.1016/j.neures.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J-A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 96.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, et al. S-Equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora1–4. Am J Clin Nutr. 2005;81(5):1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 98.Choi EJ, Kim GH. The antioxidant activity of daidzein metabolites, O‑desmethylangolensin and equol, in HepG2 cells. Mol Med Rep. 2014;9(1):328–332. doi: 10.3892/mmr.2013.1752. [DOI] [PubMed] [Google Scholar]
- 99.Lund TD, Munson DJ, Haldy ME, Setchell KD, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod. 2004;70(4):1188–1195. doi: 10.1095/biolreprod.103.023713. [DOI] [PubMed] [Google Scholar]
- 100.Gorzkiewicz J, Bartosz G, Sadowska-Bartosz I. The potential effects of phytoestrogens: the role in Neuroprotection. Molecules. 2021;26(10):2954. doi: 10.3390/molecules26102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Paula ML, Rodrigues DH, Teixeira HC, Barsante MM, Souza MA, Ferreira AP. Genistein down-modulates pro-inflammatory cytokines and reverses clinical signs of experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2008;8(9):1291–1297. doi: 10.1016/j.intimp.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 102.Razeghi Jahromi S, Arrefhosseini SR, Ghaemi A, Alizadeh A, Moradi Tabriz H, Togha M. Alleviation of experimental allergic encephalomyelitis in C57BL/6 mice by soy daidzein. Iran J Allergy Asthma Immunol. 2014;13:256–264. [PubMed] [Google Scholar]
- 103.Ghimire S, Cady NM, Lehman P, Peterson SR, Shahi SK, Rashid F, Giri S, Mangalam AK. Dietary isoflavones alter gut microbiota and lipopolysaccharide biosynthesis to reduce inflammation. Gut Microbes. 2022;14(1):2127446. doi: 10.1080/19490976.2022.2127446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jensen SN, Cady NM, Shahi SK, Peterson SR, Gupta A, Gibson-Corley KN, Mangalam AK. Isoflavone diet ameliorates experimental autoimmune encephalomyelitis through modulation of gut bacteria depleted in patients with multiple sclerosis. Sci Adv. 2021;7(28). doi: 10.1126/sciadv.abd4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shrode RL, Cady N, Jensen SN, Borcherding N, Mangalam AK. Isoflavone consumption reduces inflammation through modulation of phenylalanine and lipid metabolism. Metabolomics. 2022;18(11):84. doi: 10.1007/s11306-022-01944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Su X, Gao Y, Yang R. Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells. 2022;11(15):2296. doi: 10.3390/cells11152296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hou Y, Li J, Ying S. Tryptophan metabolism and gut microbiota: a novel regulatory axis integrating the microbiome, immunity, and cancer. Metabolites. 2023;13(11):1166. doi: 10.3390/metabo13111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun M, Ma N, He T, Johnston LJ, Ma X. Tryptophan (trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit Rev Food Sci Nutr. 2020;60(10):1760–1768. doi: 10.1080/10408398.2019.1598334. [DOI] [PubMed] [Google Scholar]
- 109.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, et al. Lactobacillus reuteri induces gut intraepithelial CD4 + CD8αα + T cells. Science. 2017;357(6353):806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J, Hornby PJ. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil. 2018;30(2). doi: 10.1111/nmo.13178. [DOI] [PubMed] [Google Scholar]
- 113.Garg A, Zhao A, Erickson SL, Mukherjee S, Lau AJ, Alston L, Chang TKH, Mani S, Hirota SA. Pregnane X receptor activation attenuates inflammation-associated intestinal epithelial barrier dysfunction by inhibiting Cytokine-induced myosin Light-Chain kinase expression and c-Jun N-Terminal kinase 1/2 activation. J Pharmacol Exp Ther. 2016;359(1):91–101. doi: 10.1124/jpet.116.234096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler C, Ismagilov R, Mazmanian S, Hsiao E, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 116.Sacramento PM, Monteiro C, Dias ASO, Kasahara TM, Ferreira TB, Hygino J, Wing AC, Andrade RM, Rueda F, Sales MC, et al. Serotonin decreases the production of Th1/Th17 cytokines and elevates the frequency of regulatory CD4 + T-cell subsets in multiple sclerosis patients. Eur J Immunol. 2018;48(8):1376–1388. doi: 10.1002/eji.201847525. [DOI] [PubMed] [Google Scholar]
- 117.Wen J, Ariyannur PS, Ribeiro R, Tanaka M, Moffett JR, Kirmani BF, Namboodiri AMA, Zhang Y. Efficacy of N-Acetylserotonin and melatonin in the EAE Model of multiple sclerosis. J Neuroimmune Pharmacol. 2016;11(4):763–773. doi: 10.1007/s11481-016-9702-9. [DOI] [PubMed] [Google Scholar]
- 118.Gaetani L, Boscaro F, Pieraccini G, Calabresi P, Romani L, Di Filippo M, Zelante T. Host and microbial tryptophan metabolic profiling in multiple sclerosis. Front Immunol. 2020;11:157. doi: 10.3389/fimmu.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao C-C, Patel B, Yan R, Blain M, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sonner JK, Keil M, Falk-Paulsen M, Mishra N, Rehman A, Kramer M, Deumelandt K, Röwe J, Sanghvi K, Wolf L, et al. Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology. Nat Commun. 2019;10(1):4877. doi: 10.1038/s41467-019-12776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Montgomery TL, Eckstrom K, Lile KH, Caldwell S, Heney ER, Lahue KG, D’Alessandro A, Wargo MJ, Krementsov DN. Lactobacillus reuteri tryptophan metabolism promotes host susceptibility to CNS autoimmunity. Microbiome. 2022;10(1):198. doi: 10.1186/s40168-022-01408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pakpoor J, Schmierer K, Cuzick J, Giovannoni G, Dobson R. Estimated and projected burden of multiple sclerosis attributable to smoking and childhood and adolescent high body-mass index: a comparative risk assessment. Int J Epidemiol. 2021;49(6):2051–2057. doi: 10.1093/ije/dyaa151. [DOI] [PubMed] [Google Scholar]
- 123.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12(1):5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 124.Stampanoni Bassi M, Iezzi E, Buttari F, Gilio L, Simonelli I, Carbone F, Micillo T, De Rosa V, Sica F, Furlan R, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2020;26(10):1237–1246. doi: 10.1177/1352458519853473. [DOI] [PubMed] [Google Scholar]
- 125.Gianfrancesco MA, Acuna B, Shen L, Briggs FB, Quach H, Bellesis KH, Bernstein A, Hedstrom AK, Kockum I, Alfredsson L, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obes Res Clin Pract. 2014;8(5):e435–47. doi: 10.1016/j.orcp.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chitnis T, Graves J, Weinstock-Guttman B, Belman A, Olsen C, Misra M, Aaen G, Benson L, Candee M, Gorman M, et al. Distinct effects of obesity and puberty on risk and age at onset of pediatric MS. Ann Clin Transl Neurol. 2016;3(12):897–907. doi: 10.1002/acn3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ji Z, Wu S, Xu Y, Qi J, Su X, Shen L. Obesity promotes EAE through IL-6 and CCL-2-Mediated T cells infiltration. Front Immunol. 2019;10:1881. doi: 10.3389/fimmu.2019.01881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, Bartkowiak-Wieczorek J, Mądry E. High-fat, Western-style Diet, systemic inflammation, and gut microbiota: a narrative review. Cells. 2021;10(11):3164. doi: 10.3390/cells10113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shahi SK, Ghimire S, Lehman P, Mangalam AK. Obesity induced gut dysbiosis contributes to disease severity in an animal model of multiple sclerosis. Front Immunol. 2022;13:966417. doi: 10.3389/fimmu.2022.966417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bittencourt A, Brum PO, Ribeiro CT, Gasparotto J, Bortolin RC, de Vargas AR, Heimfarth L, de Almeida RF, Moreira JCF, de Oliveira J, et al. High fat diet-induced obesity causes a reduction in brain tyrosine hydroxylase levels and non-motor features in rats through metabolic dysfunction, neuroinflammation and oxidative stress. Nutr Neurosci. 2022;25(5):1026–1040. doi: 10.1080/1028415X.2020.1831261. [DOI] [PubMed] [Google Scholar]
- 131.Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce‐Keller AJ, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114(6):1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saiyasit N, Chunchai T, Apaijai N, Pratchayasakul W, Sripetchwandee J, Chattipakorn N, Chattipakorn SC. Chronic high-fat diet consumption induces an alteration in plasma/brain neurotensin signaling, metabolic disturbance, systemic inflammation/oxidative stress, brain apoptosis, and dendritic spine loss. Neuropeptides. 2020;82:102047. doi: 10.1016/j.npep.2020.102047. [DOI] [PubMed] [Google Scholar]
- 133.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90(1):23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 134.Rezaeimanesh N, Razeghi Jahromi S, Ghorbani Z, Beladi Moghadam N, Hekmatdoost A, Naser Moghadasi A, Azimi AR, Sahraian MA. The association between dietary sugar intake and neuromyelitis optica spectrum disorder: a case–control study. Mult Scler Relat Disord. 2019;31:112–117. doi: 10.1016/j.msard.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 135.Peterson SR, Ali S, Shrode RL, Mangalam AK. Effect of a fructose-Rich Diet on gut microbiota and immunomodulation: potential factors for multiple sclerosis. ImmunoHorizons. 2023;7(3):213–227. doi: 10.4049/immunohorizons.2300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bhargava P, Smith MD, Mische L, Harrington E, Fitzgerald KC, Martin K, Kim S, Reyes AA, Gonzalez-Cardona J, Volsko C, et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J Clin Invest. 2020;130(7):3467–3482. doi: 10.1172/JCI129401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56(6):1085–1099. doi: 10.1194/jlr.R054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 139.Kiriyama Y, Nochi H. Physiological role of bile acids modified by the gut microbiome. Microorganisms. 2021;10(1):68. doi: 10.3390/microorganisms10010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bhagwat A, Annapure US. In vitro assessment of metabolic profile of enterococcus strains of human origin. J Genet Eng Biotechnol. 2019;17(1):11. doi: 10.1186/s43141-019-0009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xiao Y, Zhao J, Zhang H, Zhai Q, Chen W. Mining genome traits that determine the different gut colonization potential of lactobacillus and bifidobacterium species. Microb Genom. 2021;7(6). doi: 10.1099/mgen.0.000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Funabashi M, Grove TL, Wang M, Varma Y, McFadden ME, Brown LC, Guo C, Higginbottom S, Almo SC, Fischbach MA, et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. 2020;582(7813):566–570. doi: 10.1038/s41586-020-2396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heinken A, Ravcheev DA, Baldini F, Heirendt L, Fleming RMT, Thiele I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome. 2019;7(1):75. doi: 10.1186/s40168-019-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3(3):1191–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Coleman JP, Hudson LL. Cloning and characterization of a conjugated bile acid hydrolase gene from Clostridium perfringens. Appl Environ Microbiol. 1995;61(7):2514–2520. doi: 10.1128/aem.61.7.2514-2520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gopal-Srivastava R, Hylemon PB. Purification and characterization of bile salt hydrolase from Clostridium perfringens. J Lipid Res. 1988;29(8):1079–1085. doi: 10.1016/S0022-2275(20)38464-9. [DOI] [PubMed] [Google Scholar]
- 147.Stenman LK, Holma R, Korpela R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol. 2012;18(9):923–929. doi: 10.3748/wjg.v18.i9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bisschop PH, Bandsma RH, Stellaard F, Ter Harmsel A, Meijer AJ, Sauerwein HP, Kuipers F, Romijn JA. Low-fat, high-carbohydrate and high-fat, low-carbohydrate diets decrease primary bile acid synthesis in humans. Am J Clin Nutr. 2004;79(4):570–576. doi: 10.1093/ajcn/79.4.570. [DOI] [PubMed] [Google Scholar]
- 149.Frommherz L, Bub A, Hummel E, Rist MJ, Roth A, Watzl B, Kulling SE. Age-related changes of plasma bile acid concentrations in healthy adults—results from the Cross-sectional KarMeN study. PLOS ONE. 2016;11(4):e0153959. doi: 10.1371/journal.pone.0153959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bhargava P, Mowry EM. Gut microbiome and multiple sclerosis. Curr Neurol Neurosci Rep. 2014;14(10):492. doi: 10.1007/s11910-014-0492-2. [DOI] [PubMed] [Google Scholar]
- 151.Mortiboys H, Furmston R, Bronstad G, Aasly J, Elliott C, Bandmann O. UDCA exerts beneficial effect on mitochondrial dysfunction in LRRK2(G2019S) carriers and in vivo. Neurology. 2015;85(10):846–852. doi: 10.1212/WNL.0000000000001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Itoh N, Itoh Y, Tassoni A, Ren E, Kaito M, Ohno A, Ao Y, Farkhondeh V, Johnsonbaugh H, Burda J, et al. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: focus on astrocytes. Proc Natl Acad Sci USA. 2018;115(2):E302–E9. doi: 10.1073/pnas.1716032115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Allaire JM, Crowley SM, Law HT, Chang S-Y, Ko H-J, Vallance BA. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39(9):677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 154.Hansen J, Gulati A, Sartor RB. The role of mucosal immunity and host genetics in defining intestinal commensal bacteria. Curr Opin Gastroenterol. 2010;26(6):564–571. doi: 10.1097/MOG.0b013e32833f1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Inczefi O, Bacsur P, Resal T, Keresztes C, Molnar T. The influence of nutrition on intestinal permeability and the microbiome in health and disease. Front Nutr. 2022;9:718710. doi: 10.3389/fnut.2022.718710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fawkner-Corbett D, Simmons A, Parikh K. Microbiome, pattern recognition receptor function in health and inflammation. Best Pract Res Clin Gastroenterol. 2017;31(6):683–691. doi: 10.1016/j.bpg.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 157.Gauthier AE, Rotjan RD, Kagan JC. Lipopolysaccharide detection by the innate immune system may be an uncommon defence strategy used in nature. Open Biol. 2022;12(10):220146. doi: 10.1098/rsob.220146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Montgomery TL, Peipert D, Krementsov DN. Modulation of multiple sclerosis risk and pathogenesis by the gut microbiota: complex interactions between host genetics, bacterial metabolism, and diet. Immunological Rev. 2024; doi: 10.1111/imr.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang J, Zhao Y, Shao F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr Opin Immunol. 2015;32:78–83. doi: 10.1016/j.coi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 160.Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ, et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395(6699):284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 161.Wang L, Wang Q, Reeves PR. The variation of O antigens in gram-negative bacteria. Subcell Biochem. 2010;53:123–152. [DOI] [PubMed] [Google Scholar]
- 162.Scott AJ, Oyler BL, Goodlett DR, Ernst RK. Lipid a structural modifications in extreme conditions and identification of unique modifying enzymes to define the Toll-like receptor 4 structure-activity relationship. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(11):1439–1450. doi: 10.1016/j.bbalip.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Whitfield C, Kaniuk N, Frirdich E. Molecular insights into the assembly and diversity of the outer core oligosaccharide in lipopolysaccharides from Escherichia coli and Salmonella. J Endotoxin Res. 2003;9(4):244–249. doi: 10.1177/09680519030090040501. [DOI] [PubMed] [Google Scholar]
- 164.Kim SJ, Kim HM. Dynamic lipopolysaccharide transfer cascade to TLR4/MD2 complex via LBP and CD14. BMB Rep. 2017;50(2):55–57. doi: 10.5483/BMBRep.2017.50.2.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bian Y, Dong Y, Sun J, Sun M, Hou Q, Lai Y, Zhang B. Protective effect of Kaempferol on LPS-Induced inflammation and barrier dysfunction in a coculture Model of intestinal epithelial cells and intestinal microvascular endothelial cells. J Agric Food Chem. 2020;68(1):160–167. doi: 10.1021/acs.jafc.9b06294. [DOI] [PubMed] [Google Scholar]
- 166.Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur T, Hämäläinen A-M, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165(4):842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mohr AE, Crawford M, Jasbi P, Fessler S, Sweazea KL. Lipopolysaccharide and the gut microbiota: considering structural variation. FEBS Lett. 2022;596(7):849–875. doi: 10.1002/1873-3468.14328. [DOI] [PubMed] [Google Scholar]
- 168.Steimle A, Michaelis L, Di Lorenzo F, Kliem T, Munzner T, Maerz JK, Schäfer A, Lange A, Parusel R, Gronbach K, et al. Weak agonistic LPS restores intestinal immune homeostasis. Mol Ther. 2019;27(11):1974–1991. doi: 10.1016/j.ymthe.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Escribano BM, Medina-Fernandez FJ, Aguilar-Luque M, Aguera E, Feijoo M, Garcia-Maceira FI, Lillo R, Vieyra-Reyes P, Giraldo AI, Luque E, et al. Lipopolysaccharide binding protein and oxidative stress in a multiple sclerosis Model. Neurotherapeutics. 2017;14(1):199–211. doi: 10.1007/s13311-016-0480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ellestad KK, Tsutsui S, Noorbakhsh F, Warren KG, Yong VW, Pittman QJ, Power C. Early life exposure to lipopolysaccharide suppresses experimental autoimmune encephalomyelitis by promoting tolerogenic dendritic cells and regulatory T cells. J Immunol. 2009;183(1):298–309. doi: 10.4049/jimmunol.0803576. [DOI] [PubMed] [Google Scholar]
- 171.Hsiao Elaine Y, McBride Sara W, Hsien S, Sharon G, Hyde Embriette R, McCue T, Codelli J, Chow J, Reisman S, Petrosino J, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. 2015;17(5):27. doi: 10.1007/s11920-015-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shahi SK, Yadav M, Ghimire S, Mangalam AK. Role of the gut microbiome in multiple sclerosis: from etiology to therapeutics. In: Sampson T. editor. International review of neurobiology. Vol. 167. United States: Academic Press; 2022. p. 185–215. [DOI] [PubMed] [Google Scholar]
- 174.Rudzki L, Maes M. The microbiota-gut-immune-glia (MGIG) axis in Major depression. Mol Neurobiol. 2020;57(10):4269–4295. doi: 10.1007/s12035-020-01961-y. [DOI] [PubMed] [Google Scholar]
- 175.Schachter J, Martel J, Lin CS, Chang CJ, Wu TR, Lu CC, Ko Y-F, Lai H-C, Ojcius DM, Young JD, et al. Effects of obesity on depression: a role for inflammation and the gut microbiota. Brain Behav Immun. 2018;69:1–8. doi: 10.1016/j.bbi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 176.Martel J, Chang S-H, Y-F K, Hwang T-L, Young JD, Ojcius DM. Gut barrier disruption and chronic disease. Trends In Endocrinol & Metab. 2022;33(4):247–265. doi: 10.1016/j.tem.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 177.Dujmovic I, Mangano K, Pekmezovic T, Quattrocchi C, Mesaros S, Stojsavljevic N, Nicoletti F, Drulovic J. The analysis of IL-1 beta and its naturally occurring inhibitors in multiple sclerosis: the elevation of IL-1 receptor antagonist and IL-1 receptor type II after steroid therapy. J Neuroimmunol. 2009;207(1):101–106. doi: 10.1016/j.jneuroim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 178.Goyal M, Khanna D, Rana PS, Khaibullin T, Martynova E, Rizvanov AA, Khaiboullina SF, Baranwal M. Computational intelligence technique for prediction of multiple sclerosis based on serum cytokines. Front Neurol. 2019;10:10. doi: 10.3389/fneur.2019.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ireland SJ, Monson NL, Davis LS. Seeking balance: potentiation and inhibition of multiple sclerosis autoimmune responses by IL-6 and IL-10. Cytokine. 2015;73(2):236–244. doi: 10.1016/j.cyto.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, Choung RS, Ju J, Sompallae R, Gibson-Corley K, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 2017;20(6):1269–1277. doi: 10.1016/j.celrep.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nouri M, Bredberg A, Weström B, Lavasani S, Lees JR. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and Can Be induced by adoptive transfer of auto-reactive T cells. PLOS ONE. 2014;9(9):e106335. doi: 10.1371/journal.pone.0106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199(7):971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Haas J, Hug A, Viehover A, Fritzsching B, Falk CS, Filser A, Vetter T, Milkova L, Korporal M, Fritz B, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35(11):3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 184.Baughman EJ, Mendoza JP, Ortega SB, Ayers CL, Greenberg BM, Frohman EM, Karandikar NJ. Neuroantigen-specific CD8+ regulatory T-cell function is deficient during acute exacerbation of multiple sclerosis. J Autoimmun. 2011;36(2):115–124. doi: 10.1016/j.jaut.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yadav M, Stephan S, Bluestone JA. Peripherally induced tregs - role in immune homeostasis and autoimmunity. Front Immunol. 2013;4:232. doi: 10.3389/fimmu.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 187.Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee D-H, May C, Wilck N, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43(4):817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 188.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal bacteroides fragilis depends on polysaccharide a expression. J Immunol. 2010;185(7):4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 189.Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, Phillips D, Weinstock GM, Fontana L, Cross AH, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018;27(6):1222–35 e6. doi: 10.1016/j.cmet.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.He B, Hoang TK, Tian X, Taylor CM, Blanchard E, Luo M, Bhattacharjee MB, Freeborn J, Park S, Couturier J, et al. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front Immunol. 2019;10:385. doi: 10.3389/fimmu.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kaskow BJ, Baecher-Allan C. Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(4):a029025. doi: 10.1101/cshperspect.a029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132(Pt 12):3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 193.Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellino P, Ferrero B, Eid P, Novelli F. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-β. Ann Neurol. 2009;65(5):499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 194.Pandiyan P, Bhaskaran N, Zou M, Schneider E, Jayaraman S, Huehn J. Microbiome dependent regulation of T(regs) and Th17 cells in mucosa. Front Immunol. 2019;10:426. doi: 10.3389/fimmu.2019.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Duc D, Vigne S, Bernier-Latmani J, Yersin Y, Ruiz F, Gaia N, Leo S, Lazarevic V, Schrenzel J, Petrova TV, et al. Disrupting myelin-specific Th17 cell gut homing confers protection in an adoptive transfer experimental autoimmune encephalomyelitis. Cell Rep. 2019;29(2):378–90 e4. doi: 10.1016/j.celrep.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 196.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 197.Machado-Santos J, Saji E, Troscher AR, Paunovic M, Liblau R, Gabriely G, Bien CG, Bauer J, Lassmann H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. 2018;141(7):2066–2082. doi: 10.1093/brain/awy151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, Elledge SJ, Niebuhr DW, Scher AI, Munger KL, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 199.Lanz TV, Brewer RC, Ho PP, Moon JS, Jude KM, Fernandez D, Fernandes RA, Gomez AM, Nadj G-S, Bartley CM, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature. 2022;603(7900):321–327. doi: 10.1038/s41586-022-04432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rojas OL, Probstel AK, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, et al. Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10. Cell. 2019;176(3):610–24 e18. doi: 10.1016/j.cell.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Probstel AK, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, Sellrie K, Bischof A, Kim K, Ramesh A, et al. Gut microbiota–specific IgA + B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. 2020;5(53). doi: 10.1126/sciimmunol.abc7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sterlin D, Larsen M, Fadlallah J, Parizot C, Vignes M, Autaa G, Dorgham K, Juste C, Lepage P, Aboab J, et al. Perturbed Microbiota/Immune homeostasis in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8(4). doi: 10.1212/NXI.0000000000000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wahls TL, Chenard CA, Snetselaar LG. Review of two popular eating plans within the multiple sclerosis community: low saturated fat and modified paleolithic. Nutrients. 2019;11(2):352. doi: 10.3390/nu11020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Swank RL, Dugan BB. Effect of low saturated fat diet in early and late cases of multiple sclerosis. Lancet. 1990;336(8706):37–39. doi: 10.1016/0140-6736(90)91533-G. [DOI] [PubMed] [Google Scholar]
- 205.Irish AK, Erickson CM, Wahls TL, Snetselaar LG, Darling WG. Randomized control trial evaluation of a modified Paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: a pilot study. Degener Neurol Neuromuscul Dis. 2017;7:1–18. doi: 10.2147/DNND.S116949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shahi SK, Freedman SN, Murra AC, Zarei K, Sompallae R, Gibson-Corley KN, Karandikar NJ, Murray JA, Mangalam AK. Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal Model of multiple sclerosis. Front Immunol. 2019;10:462. doi: 10.3389/fimmu.2019.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jiang J, Chu C, Wu C, Wang C, Zhang C, Li T, Zhai Q, Yu L, Tian F, Chen W, et al. Efficacy of probiotics in multiple sclerosis: a systematic review of preclinical trials and meta-analysis of randomized controlled trials. Food Funct. 2021;12(6):2354–2377. doi: 10.1039/D0FO03203D. [DOI] [PubMed] [Google Scholar]
- 208.Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13–23. doi: 10.1016/j.neubiorev.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zangeneh Z, Abdi-Ali A, Khamooshian K, Alvandi A, Abiri R, Taneja V. Bacterial variation in the oral microbiota in multiple sclerosis patients. PLoS One. 2021;16(11):e0260384. doi: 10.1371/journal.pone.0260384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Boussamet L, Montassier E, Mathe C, Garcia A, Morille J, Shah S, Dugast E, Wiertlewski S, Gourdel M, Bang C, et al. Investigating the metabolite signature of an altered oral microbiota as a discriminant factor for multiple sclerosis: a pilot study. Sci Rep. 2024;14(1):7786. doi: 10.1038/s41598-024-57949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hashimoto K. Emerging role of the host microbiome in neuropsychiatric disorders: overview and future directions. Mol Psychiatry. 2023;28(9):3625–3637. doi: 10.1038/s41380-023-02287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bairamian D, Sha S, Rolhion N, Sokol H, Dorothée G, Lemere CA, Krantic S. Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer’s disease. Mol Neurodegener. 2022;17(1):19. doi: 10.1186/s13024-022-00522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


