Abstract
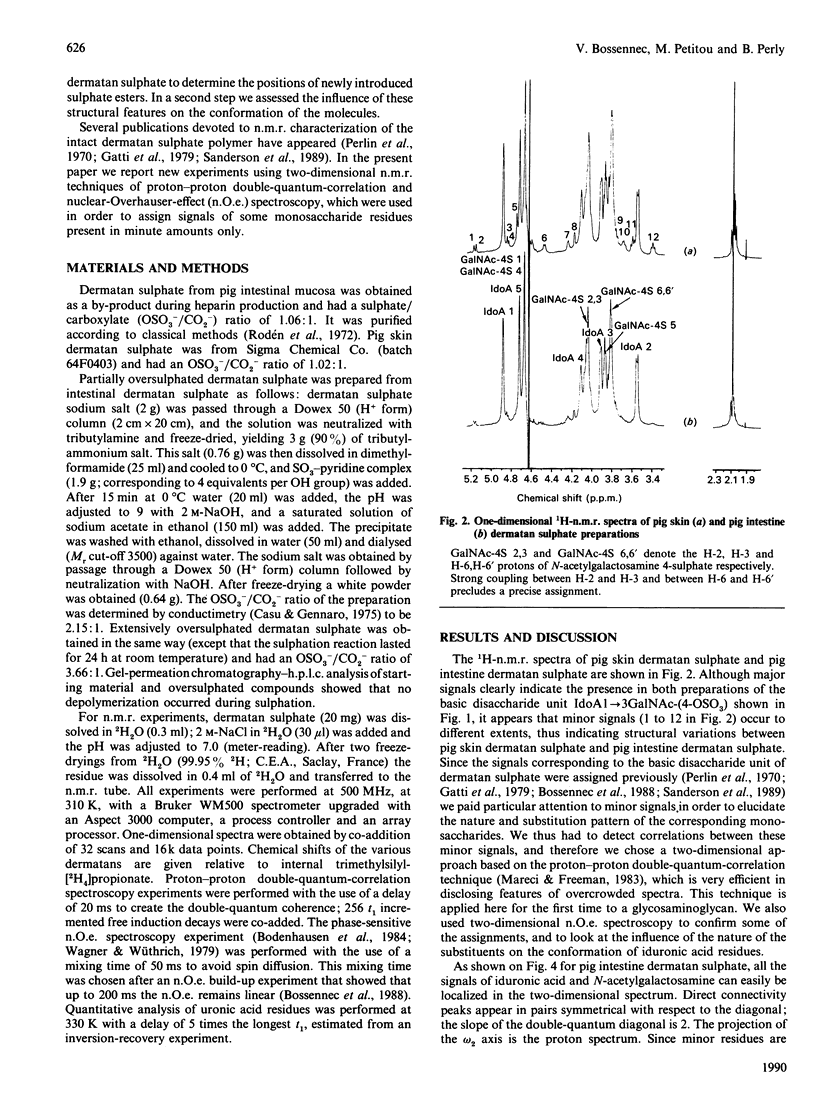
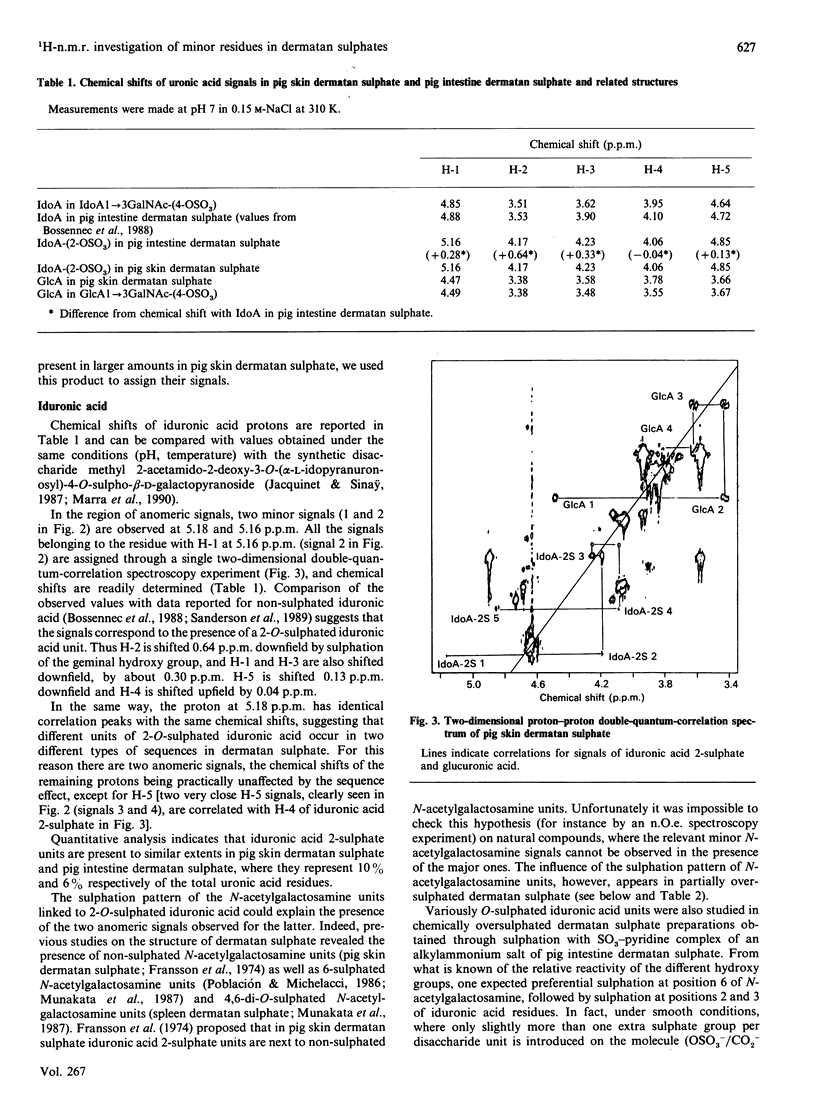
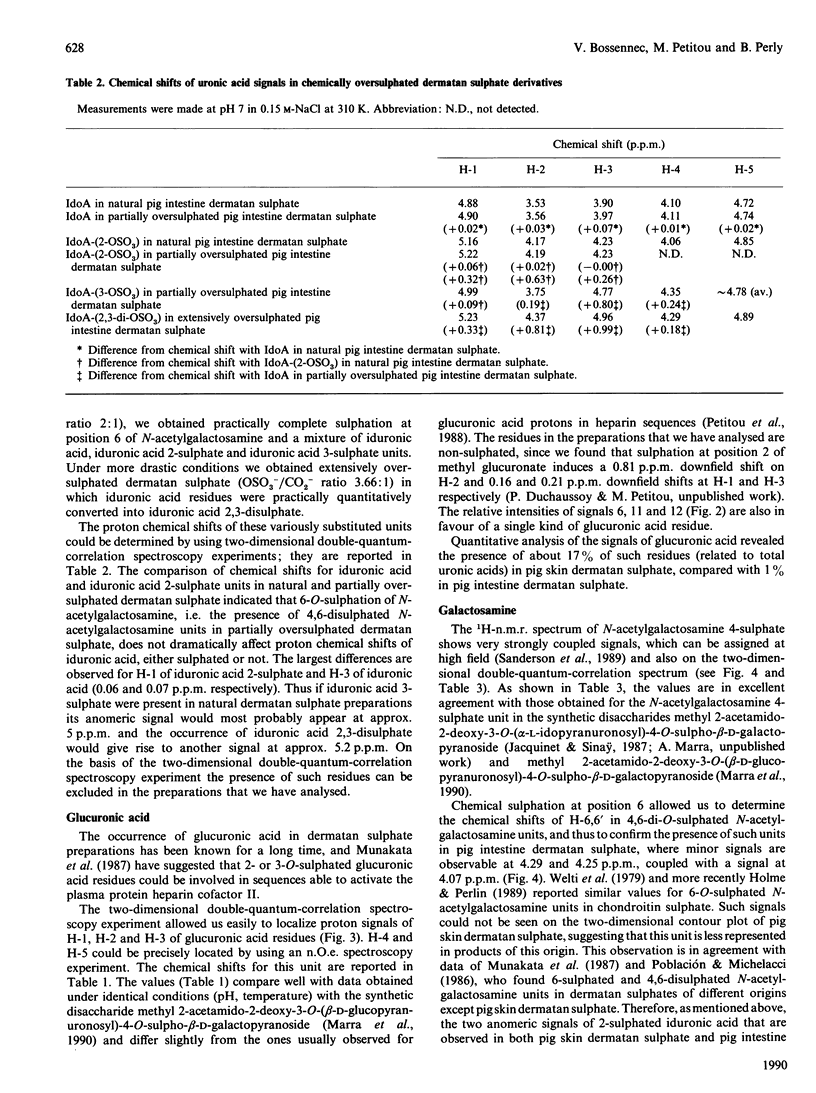
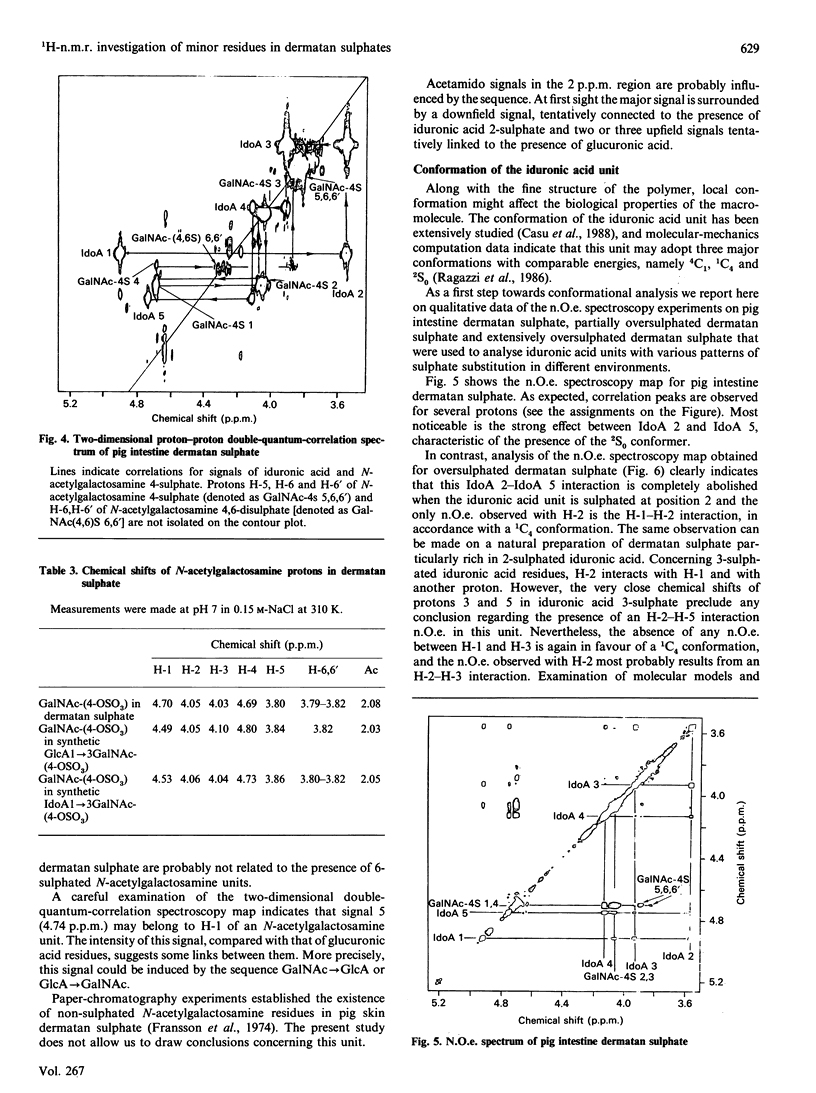
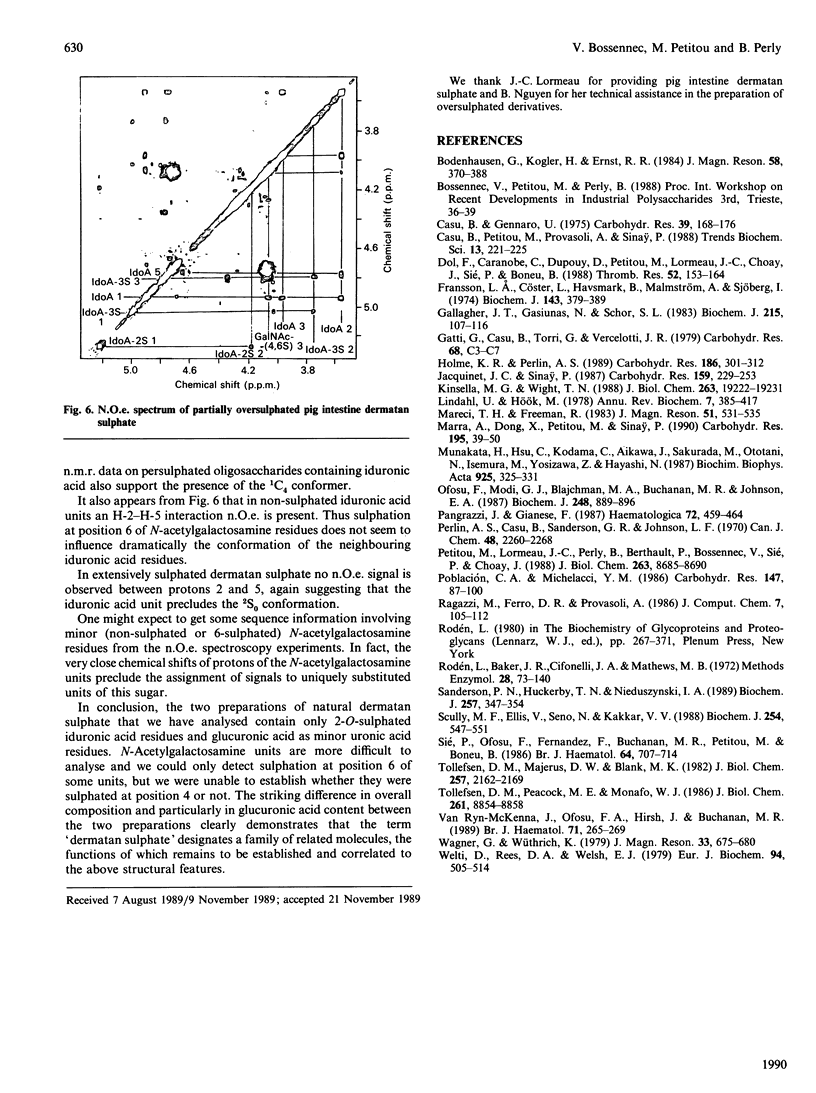
The 1H-n.m.r. spectra of various dermatan sulphate preparations present, besides the major signals of the basic disaccharide unit, several other minor signals. We have assigned most of them by n.m.r., using two-dimensional proton-proton double-quantum-correlation and nuclear-Overhauser-effect spectroscopy experiments. This allowed us to identify 2-O-sulphated L-iduronic acid and D-glucuronic acid residues as well as 6-sulphated N-acetylgalactosamine (presumably 4-O-sulphated as well). 2-O-Sulphated iduronic acid was present to similar extents (6-10% of total uronic acids) in pig skin dermatan sulphate and pig intestine dermatan sulphate, whereas glucuronic acid represented 17% of the uronic acid of pig skin dermatan sulphate and was virtually absent (1%) from the other preparation. 6-O-Sulphated N-acetylgalactosamine was present in minor amounts in pig intestine dermatan sulphate only. The influence of sulphation of iduronic acid units on their conformation was assessed by using chemically oversulphated pig intestine dermatan sulphate. Introduction of sulphate groups in this unit in dermatan sulphate tends to shift the conformational equilibrium towards the 1C4 conformer.
Full text
PDF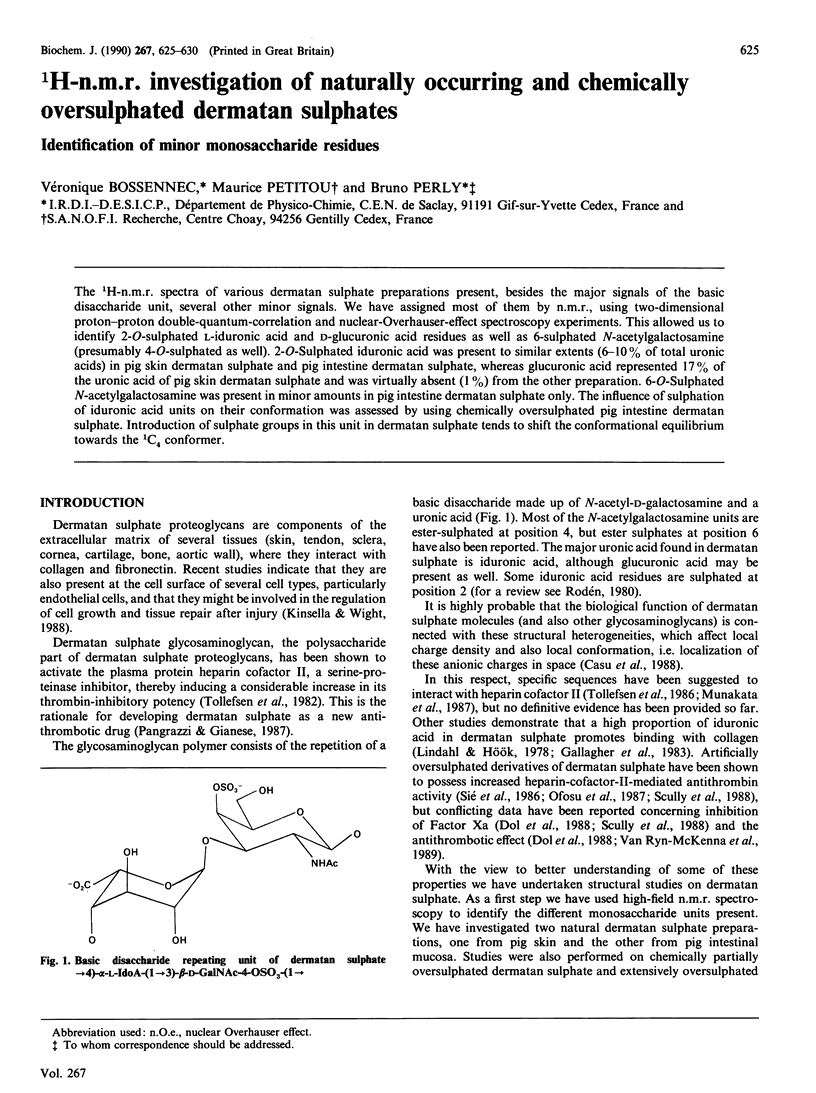
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casu B., Gennaro U. A conductimetric method for the determination of sulphate and carboxyl groups in heparin and other mucopolysaccharides. Carbohydr Res. 1975 Jan;39(1):168–176. doi: 10.1016/s0008-6215(00)82654-3. [DOI] [PubMed] [Google Scholar]
- Casu B., Petitou M., Provasoli M., Sinaÿ P. Conformational flexibility: a new concept for explaining binding and biological properties of iduronic acid-containing glycosaminoglycans. Trends Biochem Sci. 1988 Jun;13(6):221–225. doi: 10.1016/0968-0004(88)90088-6. [DOI] [PubMed] [Google Scholar]
- Dol F., Caranobe C., Dupouy D., Petitou M., Lormeau J. C., Choay J., Sié P., Boneu B. Effects of increased sulfation of dermatan sulfate on its in vitro and in vivo pharmacological properties. Thromb Res. 1988 Oct 15;52(2):153–164. doi: 10.1016/0049-3848(88)90094-1. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Cöster L., Havasmark B., Malmström A., Sjöberg I. The copolymeric structure of pig skin dermatan sulphate. Isolation and characterization of L-idurono-sulphate-containing oligosaccharides from copolymeric chains. Biochem J. 1974 Nov;143(2):379–389. doi: 10.1042/bj1430379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Gasiunas N., Schor S. L. Specific association of iduronic acid-rich dermatan sulphate with the extracellular matrix of human skin fibroblasts cultured on collagen gels. Biochem J. 1983 Oct 1;215(1):107–116. doi: 10.1042/bj2150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme K. R., Perlin A. S. Nuclear magnetic resonance spectra of heparin in admixture with dermatan sulfate and other glycosaminoglycans. 2-D spectra of the chondroitin sulfates. Carbohydr Res. 1989 Mar 15;186(2):301–312. doi: 10.1016/0008-6215(89)84044-3. [DOI] [PubMed] [Google Scholar]
- Kinsella M. G., Wight T. N. Isolation and characterization of dermatan sulfate proteoglycans synthesized by cultured bovine aortic endothelial cells. J Biol Chem. 1988 Dec 15;263(35):19222–19231. [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Marra A., Dong X., Petitou M., Sinay P. Synthesis of disaccharide fragments of dermatan sulfate. Carbohydr Res. 1989 Dec 21;195(1):39–50. doi: 10.1016/0008-6215(89)85086-4. [DOI] [PubMed] [Google Scholar]
- Munakata H., Hsu C. C., Kodama C., Aikawa J., Sakurada M., Ototani N., Isemura M., Yosizawa Z., Hayashi N. Isolation of dermatan sulfate with high heparin cofactor II-mediated thrombin-inhibitory activity from porcine spleen. Biochim Biophys Acta. 1987 Sep 11;925(3):325–331. doi: 10.1016/0304-4165(87)90198-x. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Modi G. J., Blajchman M. A., Buchanan M. R., Johnson E. A. Increased sulphation improves the anticoagulant activities of heparan sulphate and dermatan sulphate. Biochem J. 1987 Dec 15;248(3):889–896. doi: 10.1042/bj2480889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangrazzi J., Gianese F. Dermatan sulfate as a potential antithrombotic drug. Haematologica. 1987 Sep-Oct;72(5):459–464. [PubMed] [Google Scholar]
- Petitou M., Lormeau J. C., Perly B., Berthault P., Bossennec V., Sié P., Choay J. Is there a unique sequence in heparin for interaction with heparin cofactor II? Structural and biological studies of heparin-derived oligosaccharides. J Biol Chem. 1988 Jun 25;263(18):8685–8690. [PubMed] [Google Scholar]
- Población C. A., Michelacci Y. M. Structural differences of dermatan sulfates from different origins. Carbohydr Res. 1986 Mar 1;147(1):87–100. doi: 10.1016/0008-6215(86)85009-1. [DOI] [PubMed] [Google Scholar]
- Sanderson P. N., Huckerby T. N., Nieduszynski I. A. Chondroitinase ABC digestion of dermatan sulphate. N.m.r. spectroscopic characterization of the oligo- and poly-saccharides. Biochem J. 1989 Jan 15;257(2):347–354. doi: 10.1042/bj2570347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully M. F., Ellis V., Seno N., Kakkar V. V. Effect of oversulphated chondroitin and dermatan sulphate upon thrombin and factor Xa inactivation by antithrombin III or heparin cofactor II. Biochem J. 1988 Sep 1;254(2):547–551. doi: 10.1042/bj2540547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sie P., Ofosu F., Fernandez F., Buchanan M. R., Petitou M., Boneu B. Respective role of antithrombin III and heparin cofactor II in the in vitro anticoagulant effect of heparin and of various sulphated polysaccharides. Br J Haematol. 1986 Dec;64(4):707–714. doi: 10.1111/j.1365-2141.1986.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus D. W., Blank M. K. Heparin cofactor II. Purification and properties of a heparin-dependent inhibitor of thrombin in human plasma. J Biol Chem. 1982 Mar 10;257(5):2162–2169. [PubMed] [Google Scholar]
- Tollefsen D. M., Peacock M. E., Monafo W. J. Molecular size of dermatan sulfate oligosaccharides required to bind and activate heparin cofactor II. J Biol Chem. 1986 Jul 5;261(19):8854–8858. [PubMed] [Google Scholar]
- Van Ryn-McKenna J., Ofosu F. A., Hirsh J., Buchanan M. R. Antithrombotic and bleeding effects of glycosaminoglycans with different degrees of sulphation. Br J Haematol. 1989 Feb;71(2):265–269. doi: 10.1111/j.1365-2141.1989.tb04265.x. [DOI] [PubMed] [Google Scholar]
- Welti D., Rees D. A., Welsh E. J. Solution conformation of glycosaminoglycans: assignment of the 300-MHz 1H-magnetic resonance spectra of chondroitin 4-sulphate, chondroitin 6-sulphate and hyaluronate, and investigation of an alkali-induced conformation change. Eur J Biochem. 1979 Mar;94(2):505–514. doi: 10.1111/j.1432-1033.1979.tb12919.x. [DOI] [PubMed] [Google Scholar]


