Abstract
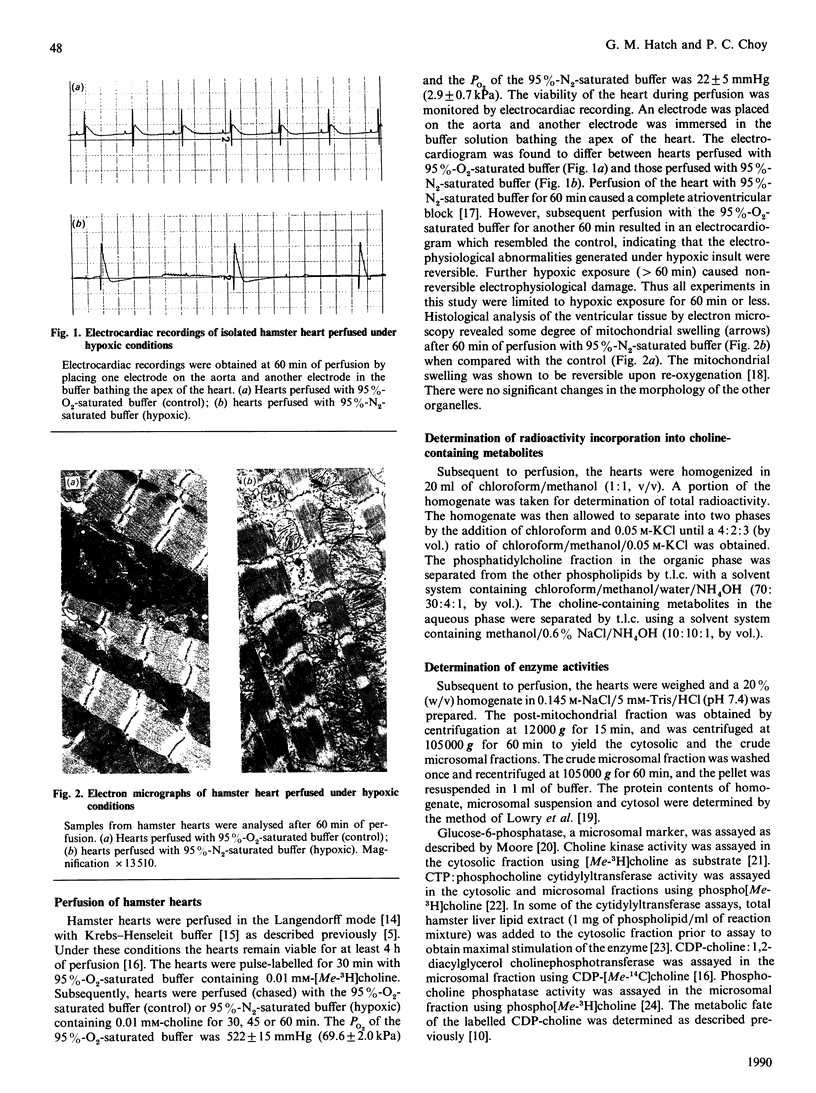
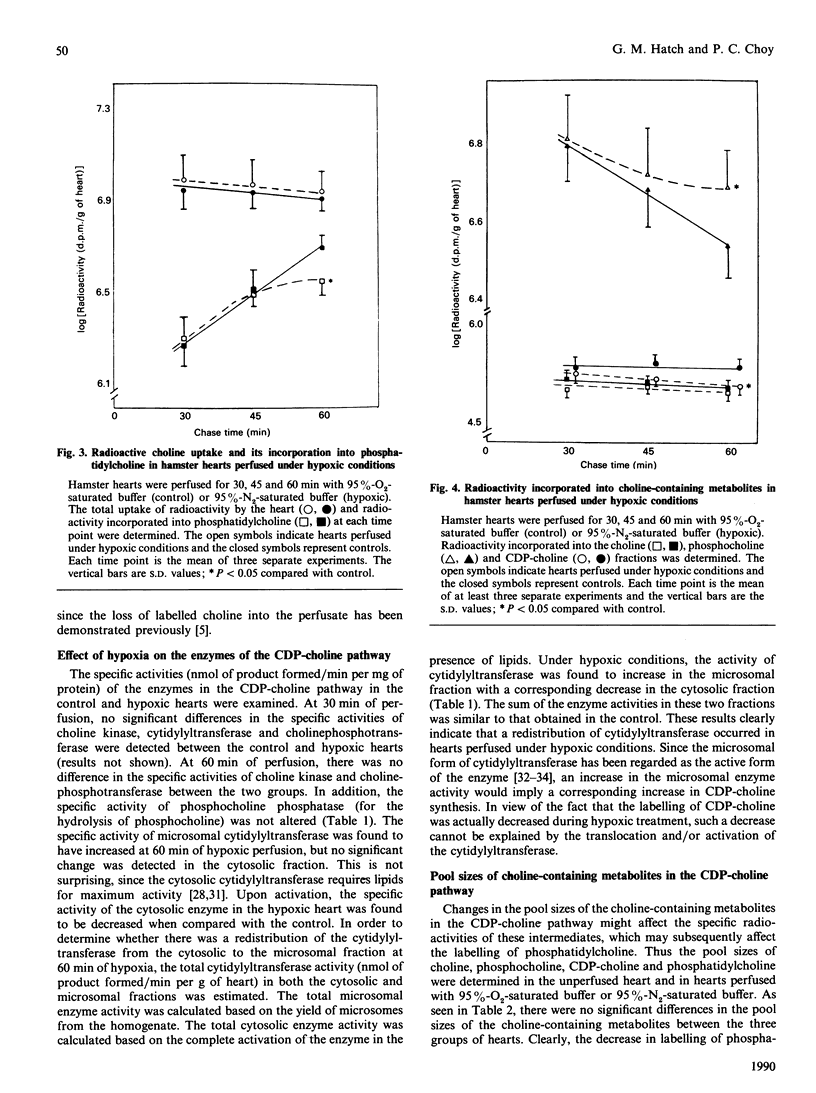
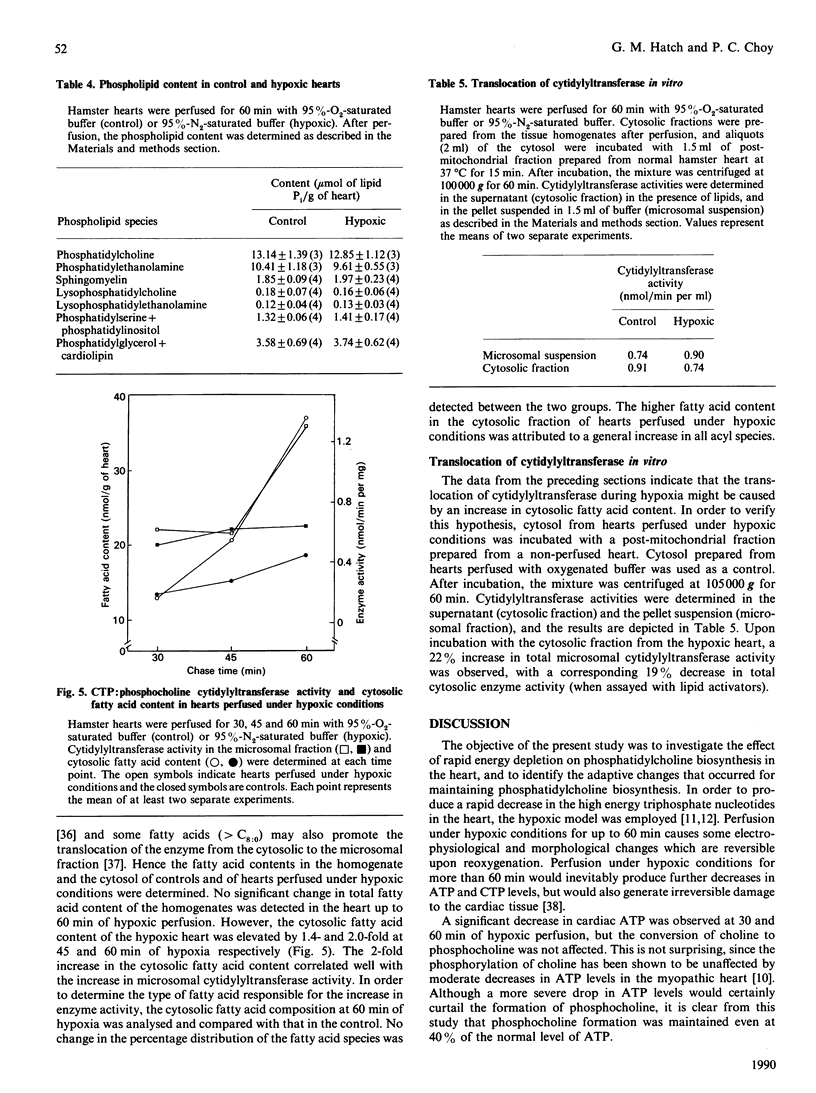
In hamster heart, the majority of the phosphatidylcholine is synthesized via the CDP-choline pathway, and the rate-limiting step of this pathway is catalysed by CTP:phosphocholine cytidylyltransferase (EC 2.7.7.15). We have shown previously [Choy (1982) J. Biol. Chem. 257, 10928-10933] that, in the myopathic heart, the level of cardiac CTP was diminished during the development of the disease. In order to maintain the level of CDP-choline, and consequently the rate of phosphatidylcholine biosynthesis, cardiac cytidylyltransferase activity was increased. However, it was not clear if the same compensatory mechanism would occur when the cardiac CTP level was decreased rapidly. In this study, hypoxia of the hamster heart was produced by perfusion with buffer saturated with 95% N2. The heart was pulse-labelled with radioactive choline and then chased with non-radioactive choline for various periods under hypoxic conditions. There was a severe decrease in ATP and CTP levels within 60 min of hypoxic perfusion, with a corresponding fall in the rate of phosphatidylcholine biosynthesis. Analysis of the choline-containing metabolites revealed that the lowered ATP level did not affect the phosphorylation of choline to phosphocholine, but the lower CTP level resulted in the decreased conversion of phosphocholine to CDP-choline. Determination of enzyme activities revealed that hypoxic treatment resulted in the enhanced translocation of cytidylyltransferase from the cytosolic to the microsomal form. This enhanced translocation was probably caused by the accumulation of fatty acids in the heart during hypoxia. We postulate that the enhancement of translocation of the cytidylyltransferase to the microsomal form (a more active form) is a mechanism by which the heart can compensate for the decrease in CTP level during hypoxia in order to maintain phosphatidylcholine biosynthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur G., Choy P. C. Acyl specificity of hamster heart CDP-choline 1,2-diacylglycerol phosphocholine transferase in phosphatidylcholine biosynthesis. Biochim Biophys Acta. 1984 Sep 12;795(2):221–229. doi: 10.1016/0005-2760(84)90069-9. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Choy P. C. Control of phosphatidylcholine biosynthesis in myopathic hamster hearts. J Biol Chem. 1982 Sep 25;257(18):10928–10933. [PubMed] [Google Scholar]
- Choy P. C., Farren S. B., Vance D. E. Lipid requirements for the aggregation of CTP:phosphocholine cytidylyltransferase in rat liver cytosol. Can J Biochem. 1979 Jun;57(6):605–612. doi: 10.1139/o79-076. [DOI] [PubMed] [Google Scholar]
- Choy P. C., Paddon H. B., Vance D. E. An increase in cytoplasmic CTP accelerates the reaction catalyzed by CTP:phosphocholine cytidylyltransferase in poliovirus-infected HeLa cells. J Biol Chem. 1980 Feb 10;255(3):1070–1073. [PubMed] [Google Scholar]
- Choy P. C., Vance D. E. Lipid requirements for activation of CTP: phosphocholine cytidylyltransferase from rat liver. J Biol Chem. 1978 Jul 25;253(14):5163–5167. [PubMed] [Google Scholar]
- Coleman R. Membrane-bound enzymes and membrane ultrastructure. Biochim Biophys Acta. 1973 Apr 3;300(1):1–30. doi: 10.1016/0304-4157(73)90010-5. [DOI] [PubMed] [Google Scholar]
- Cornell R., Vance D. E. Translocation of CTP: phosphocholine cytidylyltransferase from cytosol to membranes in HeLa cells: stimulation by fatty acid, fatty alcohol, mono- and diacylglycerol. Biochim Biophys Acta. 1987 May 13;919(1):26–36. doi: 10.1016/0005-2760(87)90214-1. [DOI] [PubMed] [Google Scholar]
- Feldman D. A., Brubaker P. G., Weinhold P. A. Activation of CTP : phosphocholine cytidylyltransferase in rat lung by fatty acids. Biochim Biophys Acta. 1981 Jul 24;665(1):53–59. doi: 10.1016/0005-2760(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Feldman D. A., Kovac C. R., Dranginis P. L., Weinhold P. A. The role of phosphatidylglycerol in the activation of CTP:phosphocholine cytidylyltransferase from rat lung. J Biol Chem. 1978 Jul 25;253(14):4980–4986. [PubMed] [Google Scholar]
- George T. P., Morash S. C., Cook H. W., Byers D. M., Palmer F. B., Spence M. W. Phosphatidylcholine biosynthesis in cultured glioma cells: evidence for channeling of intermediates. Biochim Biophys Acta. 1989 Aug 22;1004(3):283–291. doi: 10.1016/0005-2760(89)90075-1. [DOI] [PubMed] [Google Scholar]
- Hatch G. M., Choy P. C. Enhancement of choline uptake by glycine in hamster heart. Biochim Biophys Acta. 1986 Nov 19;884(2):259–264. doi: 10.1016/0304-4165(86)90171-6. [DOI] [PubMed] [Google Scholar]
- Hatch G. M., Choy P. C. Phosphocholine phosphatase and alkaline phosphatase are different enzymes in hamster heart. Lipids. 1987 Sep;22(9):672–676. doi: 10.1007/BF02533949. [DOI] [PubMed] [Google Scholar]
- Hatch G. M., Stevens W. K., Choy P. C. Effect of amino acids on choline uptake and phosphatidylcholine biosynthesis in the isolated hamster heart. Biochem Cell Biol. 1988 May;66(5):418–424. doi: 10.1139/o88-050. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowe J. E., Hawkins H. K., Jennings R. B. Relationship between depletion of high-energy phosphate compounds and lethal ischemic myocardial cell injury. Surg Forum. 1978;29:247–250. [PubMed] [Google Scholar]
- McCaman R. E., Stetzler J. Radiochemical assay for ACh: modifications for sub-picomole measurements. J Neurochem. 1977 Mar;28(3):669–671. doi: 10.1111/j.1471-4159.1977.tb10442.x. [DOI] [PubMed] [Google Scholar]
- Mock T., Slater T. L., Arthur G., Chan A. C., Choy P. C. Effects of fatty acids on phosphatidylcholine biosynthesis in isolated hamster heart. Biochem Cell Biol. 1986 May;64(5):413–417. doi: 10.1139/o86-058. [DOI] [PubMed] [Google Scholar]
- Paddon H. B., Vance D. E. The relationship between cholinephosphate phosphatase (alkaline phosphatase) and phosphatidylcholine biosynthesis in HeLa cells. Biochim Biophys Acta. 1977 Aug 24;488(2):181–189. doi: 10.1016/0005-2760(77)90175-8. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Cook H. W., Paddon H. B., Vance D. E. Membrane-bound CTP:phosphocholine cytidylyltransferase regulates the rate of phosphatidylcholine synthesis in HeLa cells treated with unsaturated fatty acids. Biochim Biophys Acta. 1984 Oct 4;795(3):433–440. doi: 10.1016/0005-2760(84)90169-3. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Pritchard P. H., Brindley D. N., Vance D. E. Fatty acids promote translocation of CTP:phosphocholine cytidylyltransferase to the endoplasmic reticulum and stimulate rat hepatic phosphatidylcholine synthesis. J Biol Chem. 1983 Jun 10;258(11):6782–6788. [PubMed] [Google Scholar]
- Pelech S. L., Vance D. E. Regulation of phosphatidylcholine biosynthesis. Biochim Biophys Acta. 1984 Jun 25;779(2):217–251. doi: 10.1016/0304-4157(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Vance D. E. Regulation of rat liver cytosolic CTP: phosphocholine cytidylyltransferase by phosphorylation and dephosphorylation. J Biol Chem. 1982 Dec 10;257(23):14198–14202. [PubMed] [Google Scholar]
- Piper H. M., Schwartz P., Hütter J. F., Spieckermann P. G. Energy metabolism and enzyme release of cultured adult rat heart muscle cells during anoxia. J Mol Cell Cardiol. 1984 Nov;16(11):995–1007. doi: 10.1016/s0022-2828(84)80013-9. [DOI] [PubMed] [Google Scholar]
- Scheuer J. Myocardial metabolism in cardiac hypoxia. Am J Cardiol. 1967 Mar;19(3):385–392. doi: 10.1016/0002-9149(67)90452-3. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Tani Y., Yamada H., Tabata M., Murachi T. Enzymatic determination of serum-free fatty acids: a colorimetric method. Anal Biochem. 1980 Sep 1;107(1):193–198. doi: 10.1016/0003-2697(80)90511-4. [DOI] [PubMed] [Google Scholar]
- Sleight R., Kent C. Regulation of phosphatidylcholine biosynthesis in mammalian cells. III. Effects of alterations in the phospholipid compositions of Chinese hamster ovary and LM cells on the activity and distribution of CTP:phosphocholine cytidylyltransferase. J Biol Chem. 1983 Jan 25;258(2):836–839. [PubMed] [Google Scholar]
- Vance D. E., Pelech S. D., Choy P. C. CTP: phosphocholine cytidylyltransferase from rat liver. Methods Enzymol. 1981;71(Pt 100):576–581. doi: 10.1016/0076-6879(81)71070-x. [DOI] [PubMed] [Google Scholar]
- Vance D. E., Trip E. M., Paddon H. B. Poliovirus increases phosphatidylcholine biosynthesis in HeLa cells by stimulation of the rate-limiting reaction catalyzed by CTP: phosphocholine cytidylyltransferase. J Biol Chem. 1980 Feb 10;255(3):1064–1069. [PubMed] [Google Scholar]
- Weinhold P. A., Rethy V. B. The separation, purification, and characterization of ethanolamine kinase and choline kinase from rat liver. Biochemistry. 1974 Dec 3;13(25):5135–5141. doi: 10.1021/bi00722a013. [DOI] [PubMed] [Google Scholar]
- Weinhold P. A., Rounsifer M. E., Williams S. E., Brubaker P. G., Feldman D. A. CTP:phosphorylcholine cytidylyltransferase in rat lung. The effect of free fatty acids on the translocation of activity between microsomes and cytosol. J Biol Chem. 1984 Aug 25;259(16):10315–10321. [PubMed] [Google Scholar]
- Whitlon D. S., Anderson K. E., Mueller G. C. Analysis of the effects of fatty acids and related compounds on the synthesis of phosphatidylcholine in lymphocytes. Biochim Biophys Acta. 1985 Jul 9;835(2):369–377. doi: 10.1016/0005-2760(85)90293-0. [DOI] [PubMed] [Google Scholar]
- Zelinski T. A., Savard J. D., Man R. Y., Choy P. C. Phosphatidylcholine biosynthesis in isolated hamster heart. J Biol Chem. 1980 Dec 10;255(23):11423–11428. [PubMed] [Google Scholar]
- de Leiris J., Harding D. P., Pestre S. The isolated perfused rat heart: a model for studying myocardial hypoxia or ischaemia. Basic Res Cardiol. 1984 May-Jun;79(3):313–321. doi: 10.1007/BF01908032. [DOI] [PubMed] [Google Scholar]



