Abstract
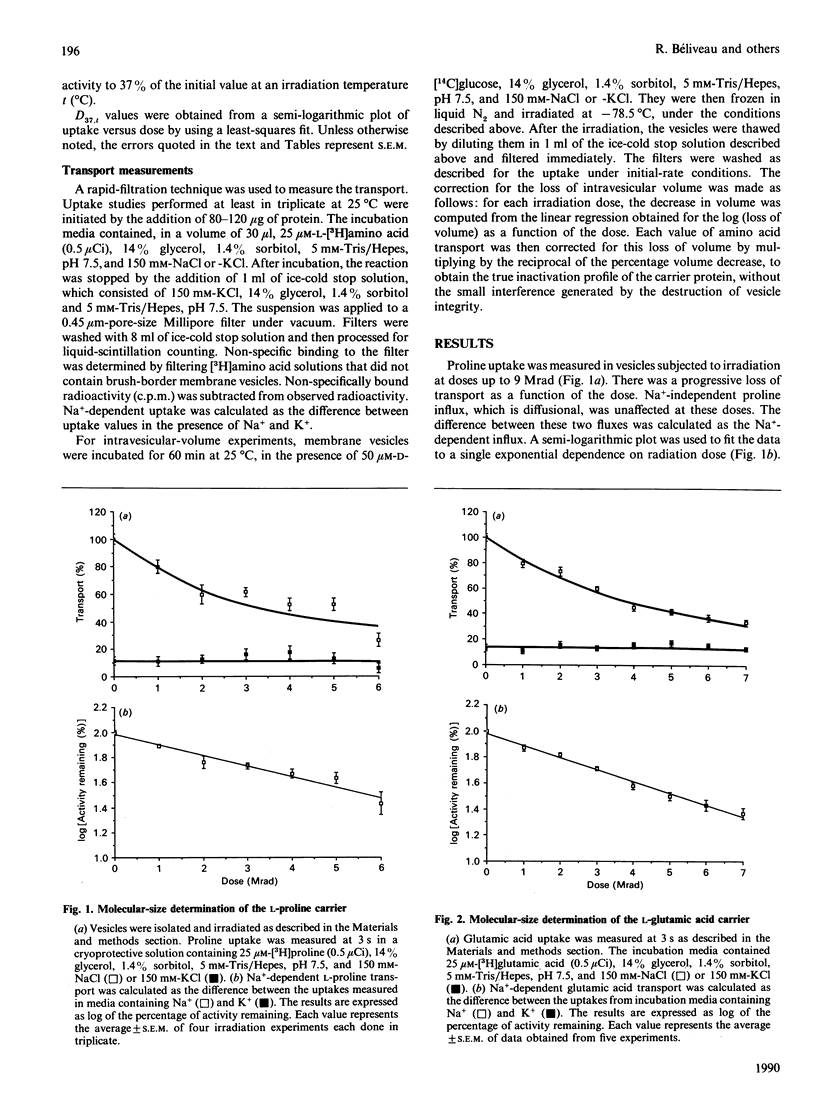
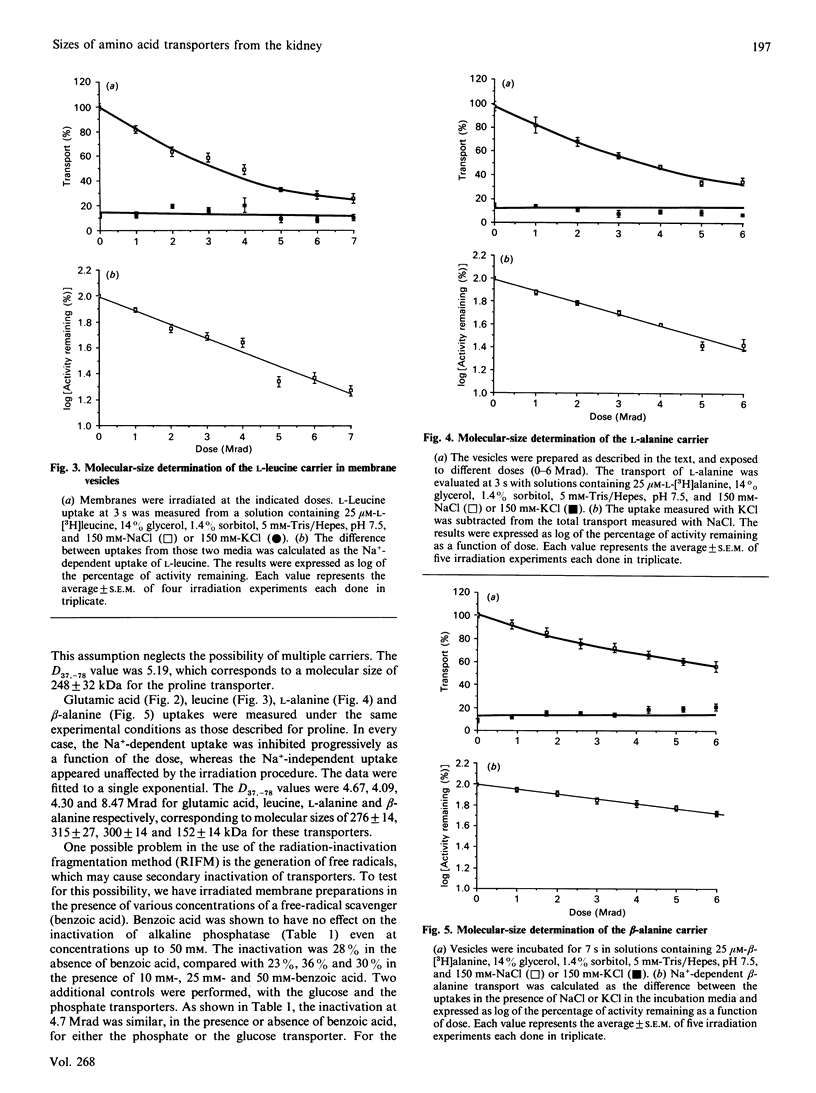
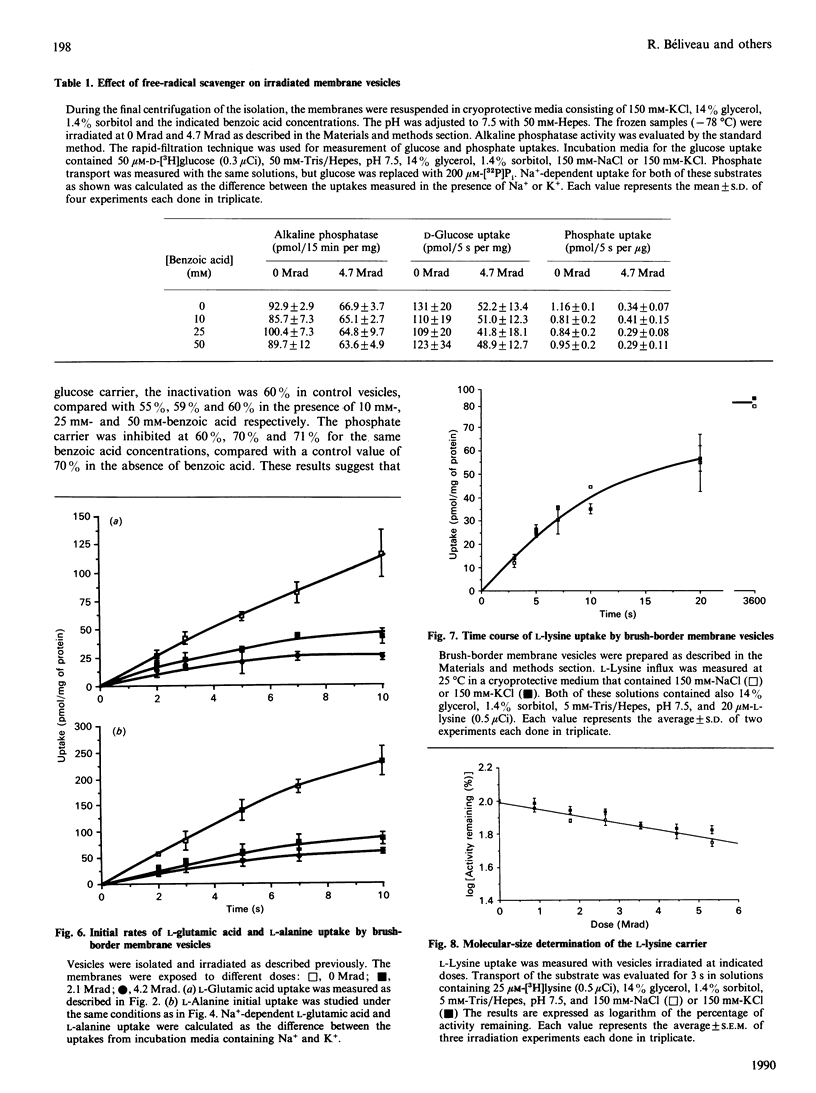
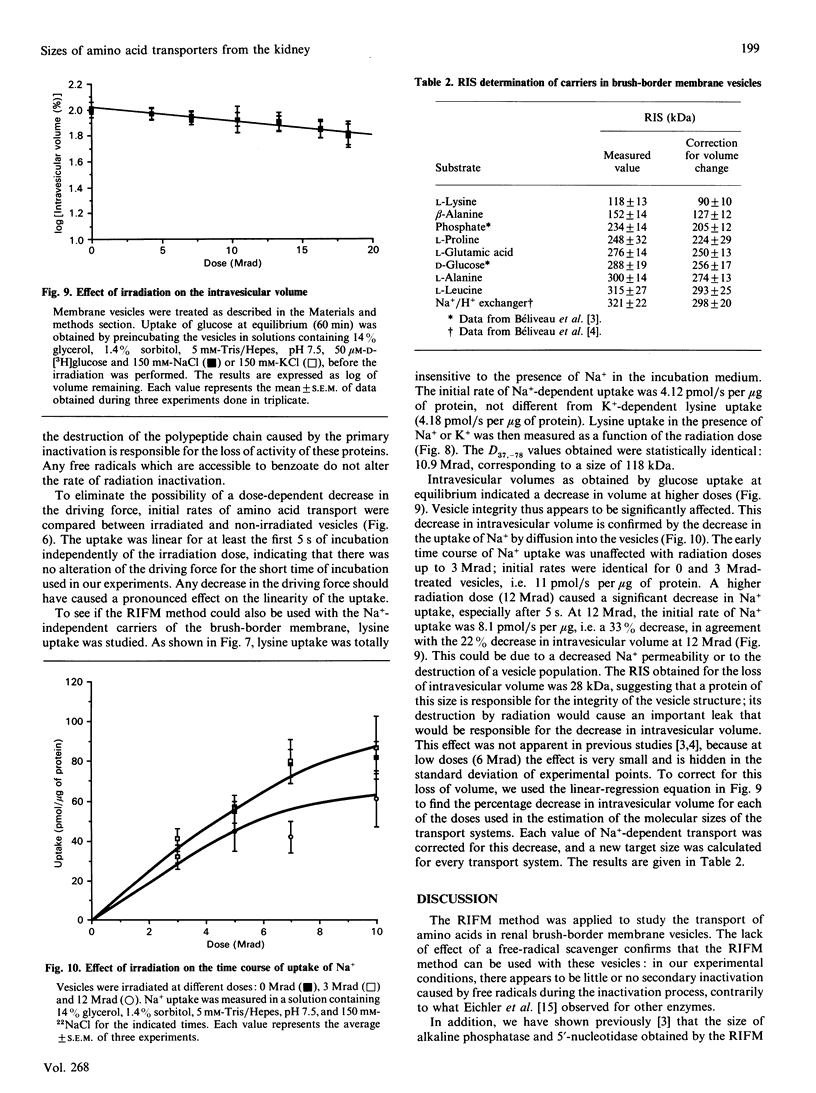
Renal brush-border membrane vesicles from rat kidney cortex were irradiated in frozen state with a gamma-radiation source. Initial rates of influx into these vesicles were estimated for substrates such as L-glutamic acid, L-alanine, L-proline and L-leucine to establish the molecular sizes of their carriers. Transport was measured in initial-rate conditions to avoid artifacts arising from a decrease in the driving force caused by a modification of membrane permeability. Initial rates of Na(+)-independent uptakes for those four substrates appeared unaffected in the dose range used (0-6 Mrad), indicating that the passive permeability of the membrane towards these substrates was unaffected. However, at higher doses of irradiation the Na+ influx and the intravesicular volume evaluated by the uptake of glucose at equilibrium were altered by radiation. Thus Na(+)-dependent influx values were corrected for volume changes, and the corrected values were used to compute radiation-inactivation sizes of the transport systems. Their respective values for L-glutamic acid, L-proline, L-leucine and L-alanine carriers were 250, 224, 293 and 274 kDa. The presence of the free-radicals scavenger benzoic acid in the frozen samples during irradiation did not affect the uptake of glucose, phosphate and alkaline phosphatase activity. These results indicate that freezing samples in a cryoprotective medium was enough to prevent secondary inactivation of transporters by free radicals. Uptakes of beta-alanine and L-lysine were much less affected by radiation. The radiation-inactivation size of the Na(+)-dependent beta-alanine carrier was 127 kDa and that of the L-lysine carrier was 90 kDa.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béliveau R., Demeule M., Ibnoul-Khatib H., Bergeron M., Beauregard G., Potier M. Radiation-inactivation studies on brush-border-membrane vesicles. General considerations, and application to the glucose and phosphate carriers. Biochem J. 1988 Jun 15;252(3):807–813. doi: 10.1042/bj2520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béliveau R., Demeule M., Potier M. Molecular size of the Na+-H+ antiport in renal brush border membranes, as estimated by radiation inactivation. Biochem Biophys Res Commun. 1988 Apr 15;152(1):484–489. doi: 10.1016/s0006-291x(88)80739-3. [DOI] [PubMed] [Google Scholar]
- Chesney R. W., Gusowski N., Dabbagh S., Theissen M., Padilla M., Diehl A. Factors affecting the transport of beta-amino acids in rat renal brush-border membrane vesicles. The role of external chloride. Biochim Biophys Acta. 1985 Feb 14;812(3):702–712. doi: 10.1016/0005-2736(85)90264-0. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. On the strategy of kinetic discrimination of amino acid transport systems. J Membr Biol. 1985;84(2):97–103. doi: 10.1007/BF01872207. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Organic ion transport during seven decades. The amino acids. Biochim Biophys Acta. 1984 Sep 3;779(3):255–269. doi: 10.1016/0304-4157(84)90012-1. [DOI] [PubMed] [Google Scholar]
- Collarini E. J., Oxender D. L. Mechanisms of transport of amino acids across membranes. Annu Rev Nutr. 1987;7:75–90. doi: 10.1146/annurev.nu.07.070187.000451. [DOI] [PubMed] [Google Scholar]
- Eichler D. C., Solomonson L. P., Barber M. J., McCreery M. J., Ness G. C. Radiation inactivation analysis of enzymes. Effect of free radical scavengers on apparent target sizes. J Biol Chem. 1987 Jul 15;262(20):9433–9436. [PubMed] [Google Scholar]
- Evers J., Murer H., Kinne R. Phenylalanine uptake in isolated renal brush border vesicles. Biochim Biophys Acta. 1976 Apr 5;426(4):598–615. doi: 10.1016/0005-2736(76)90124-3. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Sacktor B. Transport of amino acids in renal brush border membrane vesicles. Uptake of L-proline. J Biol Chem. 1977 Jan 25;252(2):591–595. [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Schlegel W. Size determination of enzymes by radiation inactivation. Anal Biochem. 1979 Jan 1;92(1):2–10. doi: 10.1016/0003-2697(79)90617-1. [DOI] [PubMed] [Google Scholar]
- Kepner G. R., Macey R. I. Membrane enzyme systems. Molecular size determinations by radiation inactivation. Biochim Biophys Acta. 1968 Sep 17;163(2):188–203. doi: 10.1016/0005-2736(68)90097-7. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Pritchard J. B. Proton-coupled L-lysine uptake by renal brush border membrane vesicles from mullet (Mugil cephalus). J Membr Biol. 1983;75(2):171–178. doi: 10.1007/BF01995635. [DOI] [PubMed] [Google Scholar]
- Lynch A. M., McGivan J. D. Evidence for a single common Na+-dependent transport system for alanine, glutamine, leucine and phenylalanine in brush-border membrane vesicles from bovine kidney. Biochim Biophys Acta. 1987 May 29;899(2):176–184. doi: 10.1016/0005-2736(87)90398-1. [DOI] [PubMed] [Google Scholar]
- Medow M. S., Roth K. S., Ginkinger K., Segal S. Renal brush-border-membrane vesicles prepared from newborn rats by free-flow electrophoresis and their proline uptake. Biochem J. 1983 Jul 15;214(1):209–214. doi: 10.1042/bj2140209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mircheff A. K., Kippen I., Hirayama B., Wright E. M. Delineation of sodium-stimulated amino acid transport pathways in rabbit kidney brush border vesicles. J Membr Biol. 1982;64(1-2):113–122. doi: 10.1007/BF01870773. [DOI] [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Evidence for tyrosyl residues at the Na+ site on the intestinal Na+/glucose cotransporter. J Biol Chem. 1985 May 25;260(10):6026–6031. [PubMed] [Google Scholar]
- Turner R. J. beta-Amino acid transport across the renal brush-border membrane is coupled to both Na and Cl. J Biol Chem. 1986 Dec 5;261(34):16060–16066. [PubMed] [Google Scholar]


