Abstract
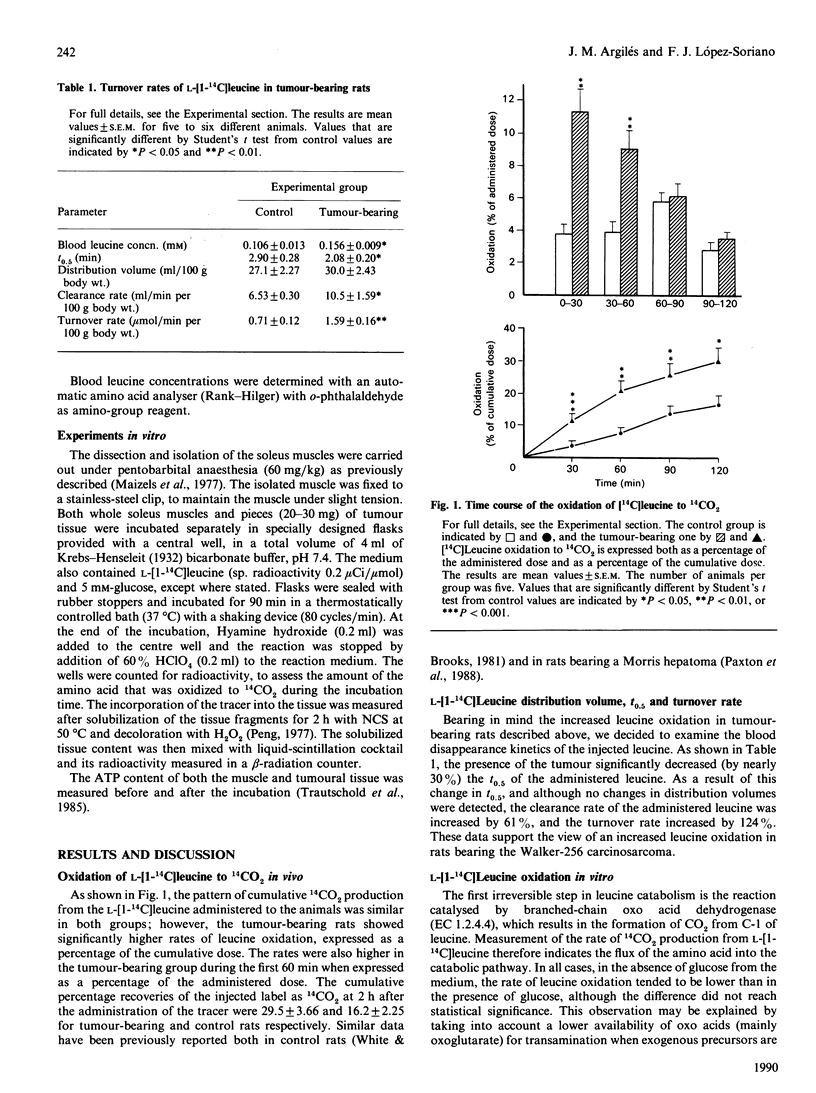
Rats bearing the Walker-256 carcinosarcoma showed significant changes in leucine metabolism compared with their non-tumour-bearing controls. After a single intravenous tracer dose of L-[1-14C]leucine in vivo, 14CO2 release by tumour-bearing rats was significantly elevated throughout the time course of administration. In addition, both the clearance and turnover rates of the tracer were significantly enhanced in these animals. Incubation of soleus muscles from control and tumour-bearing rats in the presence of L-[1-14C]leucine revealed an enhanced oxidation of the amino acid in the tumour-bearing group. Tumour tissue slices were also able to oxidize the tracer at a similar rate to that found in soleus muscles from control animals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argilés J. M., Azcón-Bieto J. The metabolic environment of cancer. Mol Cell Biochem. 1988 May;81(1):3–17. doi: 10.1007/BF00225648. [DOI] [PubMed] [Google Scholar]
- Argilés J. M., López-Soriano F. J. The effects of tumour necrosis factor-alpha (cachectin) and tumour growth on hepatic amino acid utilization in the rat. Biochem J. 1990 Feb 15;266(1):123–126. doi: 10.1042/bj2660123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse M. G., Herlong H. F., Weigand D. A. The effect of diabetes, insulin, and the redox potential on leucine metabolism by isolated rat hemidiaphragm. Endocrinology. 1976 May;98(5):1166–1175. doi: 10.1210/endo-98-5-1166. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Greengard O. The dedifferentiated pattern of enzymes in livers of tumor-bearing rats. Cancer Res. 1972 Sep;32(9):1826–1832. doi: 10.2172/4649739. [DOI] [PubMed] [Google Scholar]
- Lazo P. A. Tumour-host metabolic interaction and cachexia. FEBS Lett. 1985 Aug 5;187(2):189–192. doi: 10.1016/0014-5793(85)81239-4. [DOI] [PubMed] [Google Scholar]
- Linder-Horowitz M., Knox W. E., Morris H. P. Glutaminase activities and growth rates of rat hepatomas. Cancer Res. 1969 Jun;29(6):1195–1199. [PubMed] [Google Scholar]
- Lundholm K., Bylund A. C., Holm J., Scherstén T. Skeletal muscle metabolism in patients with malignant tumor. Eur J Cancer. 1976 Jun;12(6):465–473. doi: 10.1016/0014-2964(76)90036-0. [DOI] [PubMed] [Google Scholar]
- Lundholm K., Edström S., Karlberg I., Ekman L., Scherstén T. Glucose turnover, gluconeogenesis from glycerol, and estimation of net glucose cycling in cancer patients. Cancer. 1982 Sep 15;50(6):1142–1150. doi: 10.1002/1097-0142(19820915)50:6<1142::aid-cncr2820500618>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Lundholm K., Ekman L., Edström S., Karlberg I., Jagenburg R., Scherstén T. Protein synthesis in liver tissue under the influence of a methylcholanthrene-induced sarcoma in mice. Cancer Res. 1979 Nov;39(11):4657–4661. [PubMed] [Google Scholar]
- MIDER G. B. Some aspects of nitrogen and energy metabolism in cancerous subjects: a review. Cancer Res. 1951 Nov;11(11):821–829. [PubMed] [Google Scholar]
- Maizels E. Z., Ruderman N. B., Goodman M. N., Lau D. Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem J. 1977 Mar 15;162(3):557–568. doi: 10.1042/bj1620557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Nnanyelugo D. O., Waterlow J. C. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem J. 1976 Apr 15;156(1):185–188. doi: 10.1042/bj1560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oller do Nascimento C. M., Williamson D. H. Evidence for conservation of dietary lipid in the rat during lactation and the immediate period after removal of the litter. Decreased oxidation of oral [1-14C]triolein. Biochem J. 1986 Oct 1;239(1):233–236. doi: 10.1042/bj2390233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton K., Ward L. C., Wilce P. A. Amino acid oxidation in the tumor-bearing rat. Cancer Biochem Biophys. 1988 May;9(4):343–351. [PubMed] [Google Scholar]
- Rivera S., Azcón-Bieto J., López-Soriano F. J., Miralpeix M., Argilés J. M. Amino acid metabolism in tumour-bearing mice. Biochem J. 1988 Jan 15;249(2):443–449. doi: 10.1042/bj2490443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980 Jan;60(1):143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- Rofe A. M., Bais R., Conyers R. A. Ketone-body metabolism in tumour-bearing rats. Biochem J. 1986 Jan 15;233(2):485–491. doi: 10.1042/bj2330485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez S. Adrenal function in cancer: relation to the evolution. Eur J Cancer. 1971 Oct;7(5):381–387. doi: 10.1016/0014-2964(71)90035-1. [DOI] [PubMed] [Google Scholar]
- Shapot V. S. On the multiform relationships between the tumor and the host. Adv Cancer Res. 1979;30:89–150. doi: 10.1016/s0065-230x(08)60895-7. [DOI] [PubMed] [Google Scholar]
- WAGLE S. R., MORRIS H. P., WEBER G. COMPARATIVE BIOCHEMISTRY OF HEPATOMAS. V. STUDIES ON AMINO ACID INCORPORATION IN LIVER TUMORS OF DIFFERENT GROWTH RATES. Cancer Res. 1963 Aug;23:1003–1007. [PubMed] [Google Scholar]
- White T. P., Brooks G. A. [U-14C]glucose, -alanine, and -leucine oxidation in rats at rest and two intensities of running. Am J Physiol. 1981 Feb;240(2):E155–E165. doi: 10.1152/ajpendo.1981.240.2.E155. [DOI] [PubMed] [Google Scholar]


